Deck 5: Chemistry of Bonding: Structure and Function of Drug Molecules
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/123
العب
ملء الشاشة (f)
Deck 5: Chemistry of Bonding: Structure and Function of Drug Molecules
1
Why is platinum commonly used in electrochemistry?
A)Because it is a good conductor
B)Because it carries current well
C)Because it is generally inert
D)Because it reacts easily
A)Because it is a good conductor
B)Because it carries current well
C)Because it is generally inert
D)Because it reacts easily
C
2
What is mitosis?
A)The process of triggering cancer
B)A function for plant cells dividing
C)A function for animal cells replicating
D)The process of plant and animal cells replicating
A)The process of triggering cancer
B)A function for plant cells dividing
C)A function for animal cells replicating
D)The process of plant and animal cells replicating
D
3
For what is Louis Pasteur famous?
A)The rabies vaccine
B)The pasteurization process
C)Both of the above.
D)Neither of the above.
A)The rabies vaccine
B)The pasteurization process
C)Both of the above.
D)Neither of the above.
C
4
How does an immunoassay function?
A)By having a small molecule fit into a larger one
B)By having a small molecule deform as it is fitted into a larger one
C)By having a large molecule fit into a smaller one
D)By having a large molecule deform as it is fitted into a smaller one
A)By having a small molecule fit into a larger one
B)By having a small molecule deform as it is fitted into a larger one
C)By having a large molecule fit into a smaller one
D)By having a large molecule deform as it is fitted into a smaller one

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
5
Why do covalent bonds form between two atoms?
A)Because the polarity difference between the two is large enough for one atom to strip an electron from the other
B)Because the polarity difference between the two is not large enough for one atom to strip an electron from the other
C)Because the electronegativity difference between the two is large enough for one atom to strip an electron from the other
D)Because the electronegativity difference between the two is not large enough for one atom to strip an electron from the other
A)Because the polarity difference between the two is large enough for one atom to strip an electron from the other
B)Because the polarity difference between the two is not large enough for one atom to strip an electron from the other
C)Because the electronegativity difference between the two is large enough for one atom to strip an electron from the other
D)Because the electronegativity difference between the two is not large enough for one atom to strip an electron from the other

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following is a tenet of valence bond theory?
A)Electrons must not be shared.
B)Orbitals must overlap.
C)Orbitals must not overlap.
D)Electrons must remain localized.
A)Electrons must not be shared.
B)Orbitals must overlap.
C)Orbitals must not overlap.
D)Electrons must remain localized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
7
When p orbitals overlap to form a single bond, what part of the orbital must be overlapped?
A)The end
B)The side
C)The middle
D)Both ends
A)The end
B)The side
C)The middle
D)Both ends

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
8
Molecular interactions depend only upon size interactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
9
What must interact for a covalent bond to form?
A)Two or more protons
B)Two or more electrons
C)Two electrons
D)None of the above.
A)Two or more protons
B)Two or more electrons
C)Two electrons
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
10
The term "valence" refers to an outer, bonding electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
11
What do the Lewis structures of all the alkali metals have in common?
A)A complete sub-shell of electrons
B)One outer electron
C)Two outer electrons
D)Three outer electrons
A)A complete sub-shell of electrons
B)One outer electron
C)Two outer electrons
D)Three outer electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
12
How does Lewis theory explain the loss or gain of electrons in an ionic bond?
A)Atoms are attempting to gain electrons.
B)Atoms are attempting to lose electrons.
C)Atoms are attempting to share valence electrons.
D)Atoms are attempting to achieve a noble gas electron configuration.
A)Atoms are attempting to gain electrons.
B)Atoms are attempting to lose electrons.
C)Atoms are attempting to share valence electrons.
D)Atoms are attempting to achieve a noble gas electron configuration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
13
What do the valence shells of all the noble gas elements have in common?
A)A large size for its row on the periodic table
B)A high ability to lose electrons
C)All have eight electrons
D)A high affinity for further electrons
A)A large size for its row on the periodic table
B)A high ability to lose electrons
C)All have eight electrons
D)A high affinity for further electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the octet rule?
A)Elements form bonds to become isoelectronic with the noble gases.
B)Elements form bonds to avoid becoming isoelectronic with the noble gases.
C)Elements do not form bonds to become isoelectronic with the noble gases.
D)Elements do not form bonds to avoid becoming isoelectronic with the noble gases.
A)Elements form bonds to become isoelectronic with the noble gases.
B)Elements form bonds to avoid becoming isoelectronic with the noble gases.
C)Elements do not form bonds to become isoelectronic with the noble gases.
D)Elements do not form bonds to avoid becoming isoelectronic with the noble gases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
15
The octet rule is quite different from the "rule of eight."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which is the correct Lewis dot structure for He?
A)He:
B)H:
C)He.
D)H.
A)He:
B)H:
C)He.
D)H.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the proper Lewis dot structure for Ca?
A)Ca:
B).Ca.
C).Ca
D)Ca
A)Ca:
B).Ca.
C).Ca
D)Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
18
The correct Lewis dot structure for potassium is what?
A).K.
B).K
C).Pt
D).Pt.
A).K.
B).K
C).Pt
D).Pt.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the correct Lewis dot structure for Se?
A)
B)
C)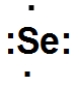
D)
A)

B)

C)
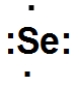
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which is the correct Lewis dot structure for chlorine?
A)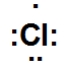
B)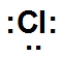
C)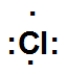
D)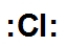
A)
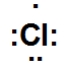
B)
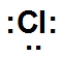
C)
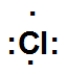
D)
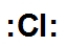

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
21
When calcium and chlorine react, they form an ionic compound. What is its Lewis dot structure?
A)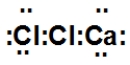
B)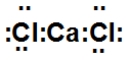
C)
D)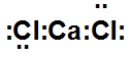
A)
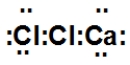
B)
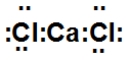
C)

D)
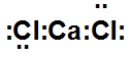

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
22
How many electrons are in the Lewis dot structure of O and S?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
23
How many valence electrons are in the Lewis dot structures of B, Si, and As?
A)3
B)4
C)5
D)None of the above.
A)3
B)4
C)5
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
24
Lewis structures with single lines indicate what kind of bonding?
A)Covalent, 2-electron bonding
B)Ionic, 2-electron bonding
C)Covalent, 4-electron bonding
D)Ionic, 4-electron bonding
A)Covalent, 2-electron bonding
B)Ionic, 2-electron bonding
C)Covalent, 4-electron bonding
D)Ionic, 4-electron bonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the correct Lewis structure for ethyne, C2H2, also known by the common name acetylene?
A)H-H-C-C
B)H-C-C-H
C)H-C=C-H
D)H-C≡C-H
A)H-H-C-C
B)H-C-C-H
C)H-C=C-H
D)H-C≡C-H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
26
The simplest covalently bonded molecule is hydrogen. Which is the correct Lewis structure?
A)H2
B)H.H
C)H == H
D)H - H
A)H2
B)H.H
C)H == H
D)H - H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which is the best Lewis structure for methane?
A)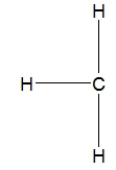
B)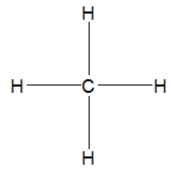
C)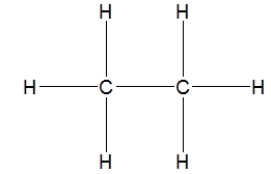
D)None of the above.
A)

B)

C)

D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which is the best representation of a water molecule?
A)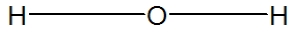
B)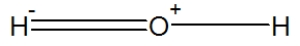
C)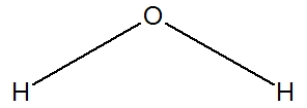
D)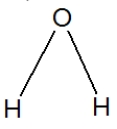
A)
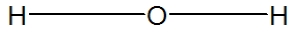
B)
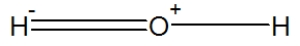
C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
29
How is ethane best represented by a Lewis structure?
A)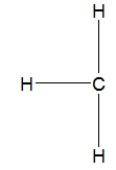
B)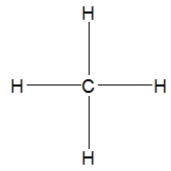
C)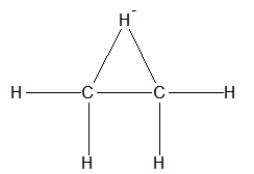
D)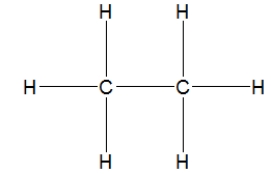
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
30
Phosphine, PH3, is structurally much like ammonia. Which of the following is the best Lewis structure of phosphine?
A)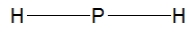
B)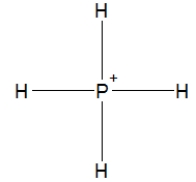
C)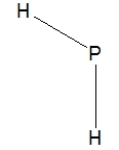
D)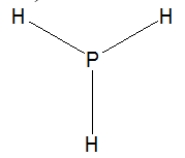
A)
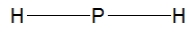
B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
31
Hydrogen sulfide is considered the "rotten egg" gas, and has a structure much like water. Which of the following is its Lewis structure?
A)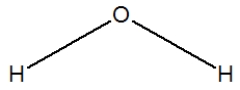
B)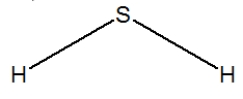
C)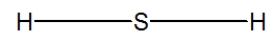
D)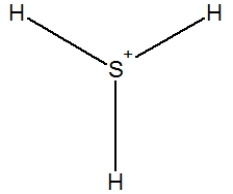
A)

B)

C)
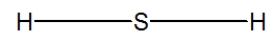
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is wrong with the Lewis structure: He=He?
A)Helium does not bond to another helium.
B)Helium only forms a single bond to a second helium.
C)Helium needs more electrons to form the bond.
D)Nothing, the bonding is correct?
A)Helium does not bond to another helium.
B)Helium only forms a single bond to a second helium.
C)Helium needs more electrons to form the bond.
D)Nothing, the bonding is correct?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
33
What does the double line, which looks much like an equals sign, represent in Lewis structures?
A)A 2-electron single bond
B)A 4-electron double bond
C)A 2-electron double bond
D)A 4-electron single bond
A)A 2-electron single bond
B)A 4-electron double bond
C)A 2-electron double bond
D)A 4-electron single bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following choices is the best Lewis structure for ammonia?
A)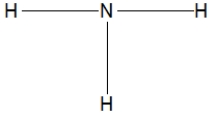
B)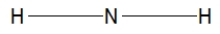
C)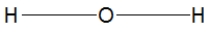
D)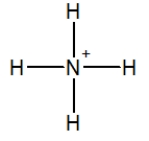
A)

B)
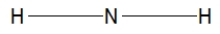
C)
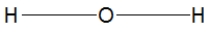
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
35
Propane, C3H8, is best represented by which condensed Lewis structure?
A)
B)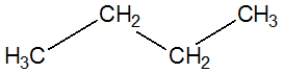
C)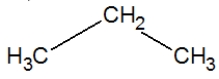
D)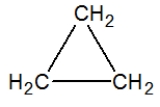
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
36
Boron trifluoride, BF3, is a corrosive material with a Lewis structure that shows boron to be two electrons short of a full octet. Which is the correct structure for BF3?
A)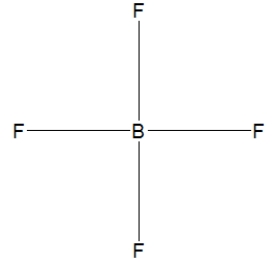
B)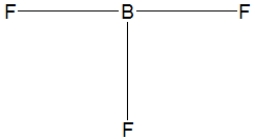
C)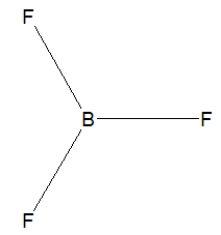
D)None of the above.
A)

B)

C)

D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the correct Lewis structure for methyl chloride, CH3Cl?
A)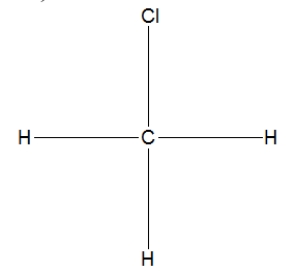
B)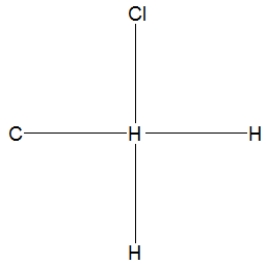
C)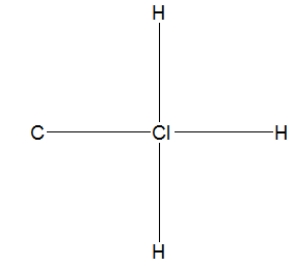
D)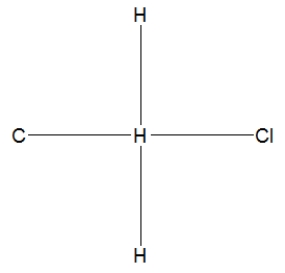
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
38
Bromine in the gaseous state is Br2 and has a simple Lewis structure. What type of bonding will it have?
A)A double, ionic bond
B)A single, ionic bond
C)A double, covalent bond
D)A single, covalent bond
A)A double, ionic bond
B)A single, ionic bond
C)A double, covalent bond
D)A single, covalent bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is wrong with this Lewis structure for gaseous hydrogen chloride: H - Cl?
A)The lone pair electrons of hydrogen are not shown here.
B)There are lone pair electrons on the chlorine which are not shown here.
C)The bond between the two atoms should be a double bond.
D)Nothing, the structure is correct.
A)The lone pair electrons of hydrogen are not shown here.
B)There are lone pair electrons on the chlorine which are not shown here.
C)The bond between the two atoms should be a double bond.
D)Nothing, the structure is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
40
Draw for yourself the Lewis structure of ethene, C2H4, also called ethylene. How many double bonds does it have?
A)None
B)1
C)2
D)3
A)None
B)1
C)2
D)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which is the name of the following compound?
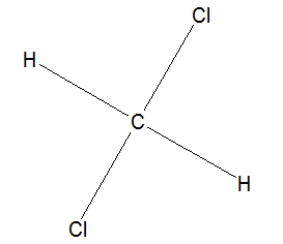
A)Chloromethane
B)Dichloromethane
C)Chloroethane
D)Dichloroethane

A)Chloromethane
B)Dichloromethane
C)Chloroethane
D)Dichloroethane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
42
After drawing the Lewis structure for the molecule ethyne, C2H2, also known by the common name acetylene, answer this: How many double bonds does it contain?
A)None
B)1
C)2
D)3
A)None
B)1
C)2
D)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
43
After drawing the correct Lewis structure for methanol, CH4O, answer this: how many single bonds does it possess?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the best structural representation of formaldehyde, CH2O?
A)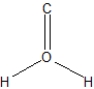
B)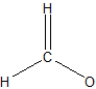
C)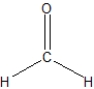
D)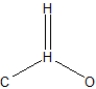
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
45
Any Lewis structure that has two lines in the same bond must contain what type of bond?
A)A single bond
B)A double bond
C)A triple bond
D)None of the above.
A)A single bond
B)A double bond
C)A triple bond
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many single bonds are in the molecule NH3?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
47
What kind of bond connects the carbon atom and oxygen atom in carbon monoxide?
A)A single bond
B)A double bond
C)A triple bond
D)Two single bonds
A)A single bond
B)A double bond
C)A triple bond
D)Two single bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
48
All covalent bonds involve the loss and gain of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
49
Draw the cyclic, organic molecule benzene, C6H6. How many resonance structures can exist for it?
A)2
B)1
C)3
D)None
A)2
B)1
C)3
D)None

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following represent the best structure for SO2?
A)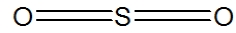
B)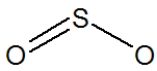
C)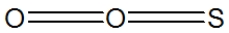
D)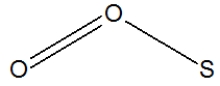
A)
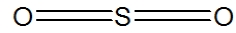
B)

C)
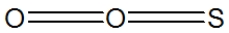
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
51
Draw the Lewis structure of ozone, O3; then ; then answer: how many resonance structures does it have?
A) None
B) 1
C) 2
D) 3
A) None
B) 1
C) 2
D) 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following would be correct structure for the nitrite ion, NO2- ?
A)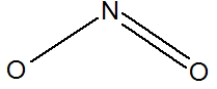
B)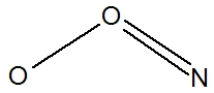
C)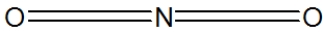
D)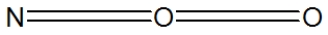
A)

B)

C)
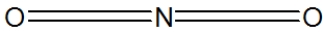
D)
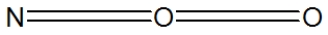

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
53
How many resonance structures can be drawn for methane?
A)2
B)3
C)4
D)None of the above
A)2
B)3
C)4
D)None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
54
What does valence shell electron pair repulsion theory help determine?
A)Atomic geometry
B)Molecular geometry
C)Molecular size
D)Atomic size
A)Atomic geometry
B)Molecular geometry
C)Molecular size
D)Atomic size

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
55
What is the shape of the water molecule?
A)Trigonal, planar
B)Planar, linear
C)Planar, bent
D)Trigonal, bent
A)Trigonal, planar
B)Planar, linear
C)Planar, bent
D)Trigonal, bent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the geometry of the very simple molecule, carbon monoxide?
A)Linear
B)Bent, planar
C)Square planar
D)Horizontal
A)Linear
B)Bent, planar
C)Square planar
D)Horizontal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
57
What shape does the ammonia molecule have?
A)Trigonal planar
B)Square planar
C)Trigonal pyramidal
D)Square pyramidal
A)Trigonal planar
B)Square planar
C)Trigonal pyramidal
D)Square pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
58
Any molecule that has a central atom with no lone pairs, and four other, equivalent atoms connected to it, has what shape?
A)Square
B)Triangular
C)Tetrahedral
D)Flat
A)Square
B)Triangular
C)Tetrahedral
D)Flat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
59
The four atoms of acetylene, C2H2, known more formally as ethyne, have what molecular shape?
A)Tetrahedral
B)Square
C)Bent
D)Linear
A)Tetrahedral
B)Square
C)Bent
D)Linear

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
60
After drawing the Lewis structure for phosphine, PH3, which should be structurally similar to ammonia, what shape should the molecule have?
A)A tetrahedron
B)A triangular pyramid
C)A square plane
D)A square pyramid
A)A tetrahedron
B)A triangular pyramid
C)A square plane
D)A square pyramid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
61
What shape should the molecule SF4 have?
A)A "saw horse" shape
B)Tetrahedron
C)Trigonal planar
D)Cubic
A)A "saw horse" shape
B)Tetrahedron
C)Trigonal planar
D)Cubic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
62
How many regions of electron density surround an atom that is the center of an octahedron?
A)8
B)4
C)6
D)12
A)8
B)4
C)6
D)12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
63
Knowing the molecule BCl3 has only three covalent bonds to the boron atom, and no electron lone pair on the boron atom, what shape does the molecule have?
A)Trigonal pyramidal
B)Trigonal planar
C)Tetrahedral
D)Linear
A)Trigonal pyramidal
B)Trigonal planar
C)Tetrahedral
D)Linear

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
64
What does molecular polarity affect?
A)Solvent interaction
B)Melting point and boiling point
C)Both of the above.
D)None of the above.
A)Solvent interaction
B)Melting point and boiling point
C)Both of the above.
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
65
As you move from the top to the bottom of the periodic table, what happens to the electronegativity of the elements?
A)It increases.
B)It decreases.
C)Nothing, it remains relatively equal.
D)More information is needed to tell.
A)It increases.
B)It decreases.
C)Nothing, it remains relatively equal.
D)More information is needed to tell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
66
What trend do the noble gases display in relation to electronegativity?
A)Electronegativity decreases as one goes down the noble gas column.
B)Electronegativity increases as one goes down the noble gas column.
C)The noble gases are all considered to have zero electronegativity.
D)For this column, there is no trend in electronegativity differences.
A)Electronegativity decreases as one goes down the noble gas column.
B)Electronegativity increases as one goes down the noble gas column.
C)The noble gases are all considered to have zero electronegativity.
D)For this column, there is no trend in electronegativity differences.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
67
How is the S-H bond categorized?
A)Polar covalent
B)Nonpolar covalent
C)Covalent
D)Ionic
A)Polar covalent
B)Nonpolar covalent
C)Covalent
D)Ionic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
68
What best describes the Cl-Cl bond?
A)Nonpolar
B)Covalent
C)Polar covalent
D)Ionic
A)Nonpolar
B)Covalent
C)Polar covalent
D)Ionic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
69
How is the N-Br bond described?
A)Pure covalent
B)Polar covalent
C)Nonpolar covalent
D)Ionic
A)Pure covalent
B)Polar covalent
C)Nonpolar covalent
D)Ionic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the electronegativity difference in the C-O bond?
A)1.0
B)2.5
C)3.5
D)0
A)1.0
B)2.5
C)3.5
D)0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
71
How great is the electronegativity difference in the average C-F bond?
A)0.5
B)1.0
C)1.5
D)2.0
A)0.5
B)1.0
C)1.5
D)2.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
72
Many organic compounds have numerous C-H bonds. What is the average difference in electronegativity in one of them?
A)4.5
B)2.5
C)2.1
D)0.4
A)4.5
B)2.5
C)2.1
D)0.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
73
What is the electronegativity difference in one of the H-Se bonds of H2Se?
A)2.4
B)2.1
C)0.4
D)0.3
A)2.4
B)2.1
C)0.4
D)0.3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
74
How can you describe the molecule CO2 in terms of polarity?
A)A polar molecule made from polar covalent bonds
B)A non-polar molecule made from polar covalent bonds
C)A non-polar molecule made from nonpolar covalent bonds
D)A polar molecule made from nonpolar covalent bonds
A)A polar molecule made from polar covalent bonds
B)A non-polar molecule made from polar covalent bonds
C)A non-polar molecule made from nonpolar covalent bonds
D)A polar molecule made from nonpolar covalent bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
75
In terms of polarity, what best describes the molecule NH3?
A)A non-polar molecule composed of polar covalent bonds
B)A polar molecule composed of nonpolar covalent bonds
C)A polar molecule composed of polar covalent bonds
D)A non-polar molecule composed of nonpolar covalent bonds
A)A non-polar molecule composed of polar covalent bonds
B)A polar molecule composed of nonpolar covalent bonds
C)A polar molecule composed of polar covalent bonds
D)A non-polar molecule composed of nonpolar covalent bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
76
How is the polarity of the molecule carbon monoxide best described?
A)A polar molecule made from a polar covalent bond
B)A non-polar molecule made from a polar covalent bond
C)A polar molecule made from a nonpolar covalent bond
D)None of the above.
A)A polar molecule made from a polar covalent bond
B)A non-polar molecule made from a polar covalent bond
C)A polar molecule made from a nonpolar covalent bond
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
77
The methane molecule can best be described in what manner, when referring to polarity?
A)A nonpolar molecule made from polar covalent bonds
B)A nonpolar molecule made from non-polar covalent bonds
C)A polar molecule made from non-polar covalent bonds
D)A polar molecule made from polar covalent bonds
A)A nonpolar molecule made from polar covalent bonds
B)A nonpolar molecule made from non-polar covalent bonds
C)A polar molecule made from non-polar covalent bonds
D)A polar molecule made from polar covalent bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
78
Of the following solvents: ethanol, water, hexane, which is the most polar?
A)Ethanol
B)Hexane
C)Water
D)They are all of roughly equal polarity.
A)Ethanol
B)Hexane
C)Water
D)They are all of roughly equal polarity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which of the following is the most polar: benzene, hexane, toluene?
A)Toluene
B)Benzene
C)Hexane
D)None of the above. They are all of equal polarity.
A)Toluene
B)Benzene
C)Hexane
D)None of the above. They are all of equal polarity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck
80
The alkane solvents are quite soluble in water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 123 في هذه المجموعة.
فتح الحزمة
k this deck








