Deck 7: Acids and Bases
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/27
العب
ملء الشاشة (f)
Deck 7: Acids and Bases
1
Identify the type of species highlighted in red in the following equation:
NH3 (aq) + HF (aq) ⇌ NH4+ (aq) + F- (aq)
Please select all that apply.
A) Arrhenius base
B) Brønsted-Lowry acid
C) Arrhenius acid
D) Brønsted-Lowry base
NH3 (aq) + HF (aq) ⇌ NH4+ (aq) + F- (aq)
Please select all that apply.
A) Arrhenius base
B) Brønsted-Lowry acid
C) Arrhenius acid
D) Brønsted-Lowry base
D
2
In water the H+ ion is always hydrated and found as the H3O+ ion.
True
3
The conjugate acid of H2PO4- is H3PO4.
True
4
Water can be described as amphoteric as it can be both a proton donor and a proton acceptor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
5
Choose which expression for the equilibrium constant for the reaction of water with perchloric (HClO4) acid is correct.
A)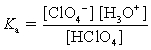
B)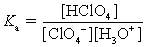
C)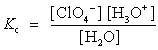
D)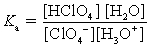
A)
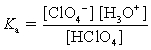
B)
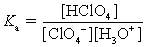
C)
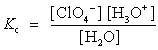
D)
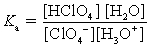

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
6
HClO4 is a strong acid, therefore ClO4- must be a _____ base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the hydrogen ion concentration of a can of cola with pH = 2.77?
A) 588 mol dm-3
B) 2.34 x 10-3 mol dm-3
C) 0.56 x 10-3 mol dm-3
D) 1.70 x 10-3 mol dm-3
A) 588 mol dm-3
B) 2.34 x 10-3 mol dm-3
C) 0.56 x 10-3 mol dm-3
D) 1.70 x 10-3 mol dm-3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
8
The pH of a 0.345 mol dm-3 solution of nitric acid is 0.46.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
9
HF has the ability to dissolve glass, it is a _____ acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following statements apply to a weak acid?
A) In the equilibrium equation:
HA (aq) + H2O (l) ⇌ A- (aq) + H3O+ (aq)
The equilibrium lies almost entirely to the right.
B) pKa is less than 0.
C) The pH would be greater than zero.
D) The pH would be less than zero.
A) In the equilibrium equation:
HA (aq) + H2O (l) ⇌ A- (aq) + H3O+ (aq)
The equilibrium lies almost entirely to the right.
B) pKa is less than 0.
C) The pH would be greater than zero.
D) The pH would be less than zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
11
Sn2+ is rapidly oxidised to Sn4+ in solution. Hydrolysis of Sn4+ ions causes the pH of the solution to rise.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
12
The pH of a 0.111 mol dm-3 HCO2H solution at 298 K is 0.95.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
13
Many solvents self ionize to form an anion and cation that are the Brønsted- Lowry base and acid of the original solvent. Match the ions with the solvent from which they are generated.
-H3PO4
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+
-H3PO4
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
14
Many solvents self ionize to form an anion and cation that are the Brønsted- Lowry base and acid of the original solvent. Match the ions with the solvent from which they are generated.
-NH3
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+
-NH3
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
15
Many solvents self ionize to form an anion and cation that are the Brønsted- Lowry base and acid of the original solvent. Match the ions with the solvent from which they are generated.
-H2SO3
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+
-H2SO3
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
16
Many solvents self ionize to form an anion and cation that are the Brønsted- Lowry base and acid of the original solvent. Match the ions with the solvent from which they are generated.
-H2O
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+
-H2O
A) H2PO4-
B) NH2-
C) H3SO3+
D) H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
17
Kw falls with temperature as the self-ionization of water is exothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
18
The position of neutral pH on the pH scale changes with temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following statements are true when referring to a buffer solution? Please select all that apply.
A) A buffer can consist of a weak acid and its salt.
B) A buffer solution is resistant to addition of small quantities of acid.
C) A buffer can consist of a weak base and its salt.
D) A buffer solution is resistant to addition of small quantities of base.
A) A buffer can consist of a weak acid and its salt.
B) A buffer solution is resistant to addition of small quantities of acid.
C) A buffer can consist of a weak base and its salt.
D) A buffer solution is resistant to addition of small quantities of base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
20
A buffer can be made from a weak base and the ____ of that base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the pH of a buffer solution containing 0.155 mol dm-3 of benzoic acid and 0.315 mol dm-3 sodium benzoate. (Ka of benzoic acid is 4.20.)
A) 0.50
B) -0.50
C) 3.89
D) 4.51
A) 0.50
B) -0.50
C) 3.89
D) 4.51

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following indicators would be suitable for the titration of HCO2H with sodium hydroxide?
A) Phenolphthalein
B) Methyl orange
C) Bromocresol blue
D) Thymol blue
A) Phenolphthalein
B) Methyl orange
C) Bromocresol blue
D) Thymol blue

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
23
The strength of an oxoacid, HmXOn containing m hydroxide groups and (n - m) double bonded oxygen atoms is related to?
A) n
B) n - m
C) n + m
D) m
A) n
B) n - m
C) n + m
D) m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
24
A polybasic acid is an acid with more than one ionisable ________ atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
25
CaO is described as being a basic oxide because it forms an alkaline solution with water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following species could be described as Lewis acids? Please select all that apply.
A) Co3+
B) F-
C) BCl3
D) I2
A) Co3+
B) F-
C) BCl3
D) I2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck
27
NH3 is a Brønsted-Lowry base and a Lewis base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 27 في هذه المجموعة.
فتح الحزمة
k this deck








