Deck 6: Principles of Stereochemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/25
العب
ملء الشاشة (f)
Deck 6: Principles of Stereochemistry
1
Examines these two molecules:
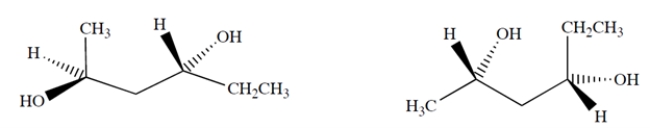 The molecules are ___________.
The molecules are ___________.
A) identical
B) enantiomers
C) diastereomers
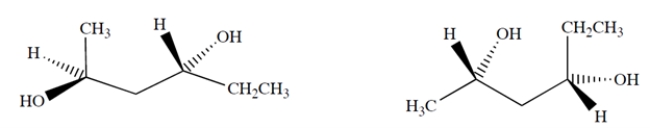 The molecules are ___________.
The molecules are ___________.A) identical
B) enantiomers
C) diastereomers
C
2
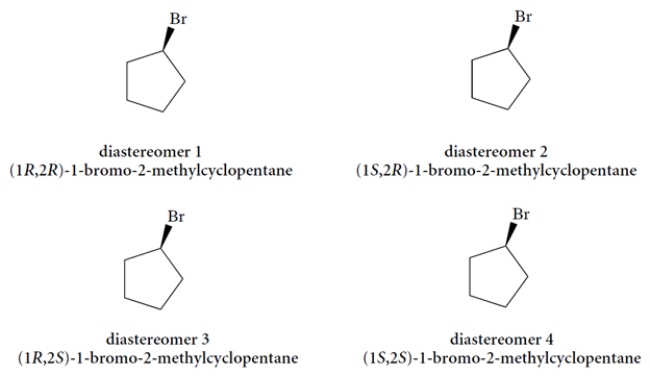
Using the templates provided, draw the four possible diastereomers of 1-bromo-2-methylcyclopentane. (You must add the methyl group at a carbon adjacent to the bromine with wedge or dashed wedge bond, as appropriate.)

You first must realize that the R or S configuration of carbon 1 is changed by moving the methyl from one adjacent carbon to the other. Thus, we have:
 This is equivalent to interchanging two groups-in this case, the ring bonds-to the asymmetric carbon. Next, pick a configuration for carbon-2 and see what it is. We'll pick the easiest one, with the methyl up.
This is equivalent to interchanging two groups-in this case, the ring bonds-to the asymmetric carbon. Next, pick a configuration for carbon-2 and see what it is. We'll pick the easiest one, with the methyl up.
 We simply switch the methyl and hydrogen positions at carbon 2 to give the other two stereoisomers.
We simply switch the methyl and hydrogen positions at carbon 2 to give the other two stereoisomers.
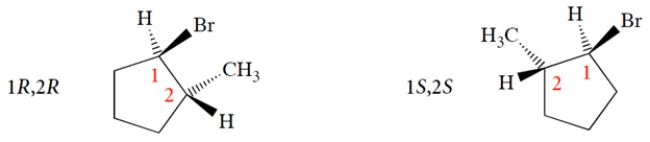 So, on your paper, the structures should be as follows. (You don't have to show the Hs, as they are assumed to have opposite stereochemistry to the Br or CH3 on the same carbon.)
So, on your paper, the structures should be as follows. (You don't have to show the Hs, as they are assumed to have opposite stereochemistry to the Br or CH3 on the same carbon.)
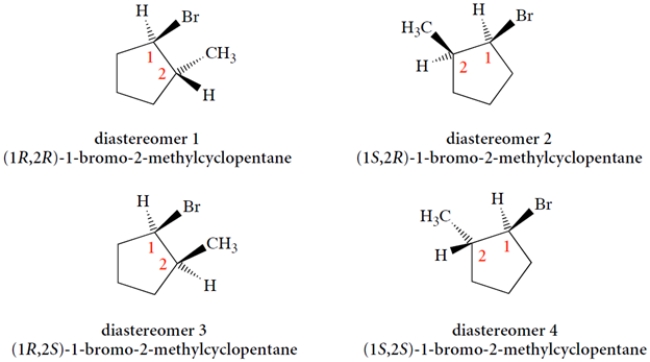
 This is equivalent to interchanging two groups-in this case, the ring bonds-to the asymmetric carbon. Next, pick a configuration for carbon-2 and see what it is. We'll pick the easiest one, with the methyl up.
This is equivalent to interchanging two groups-in this case, the ring bonds-to the asymmetric carbon. Next, pick a configuration for carbon-2 and see what it is. We'll pick the easiest one, with the methyl up. We simply switch the methyl and hydrogen positions at carbon 2 to give the other two stereoisomers.
We simply switch the methyl and hydrogen positions at carbon 2 to give the other two stereoisomers.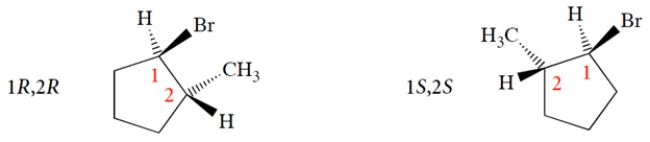 So, on your paper, the structures should be as follows. (You don't have to show the Hs, as they are assumed to have opposite stereochemistry to the Br or CH3 on the same carbon.)
So, on your paper, the structures should be as follows. (You don't have to show the Hs, as they are assumed to have opposite stereochemistry to the Br or CH3 on the same carbon.)
3
Identify whether the molecule is chiral or achiral by circling the appropriate work below the structure. Then identify any asymmetric carbons with an asterisk.
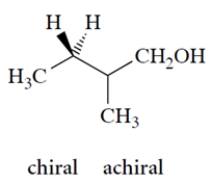

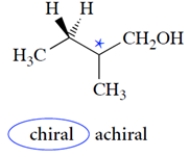
4
Determine the relationship between the two compounds.

A) constitutional isomers
B) a pair of enantiomers
C) identical molecules
D) a pair of diastereomers
E) none of these choices

A) constitutional isomers
B) a pair of enantiomers
C) identical molecules
D) a pair of diastereomers
E) none of these choices

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
5
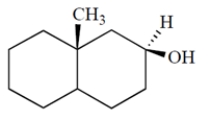
Identify the asymmetric carbons in the structure.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following structures represents a meso compound?
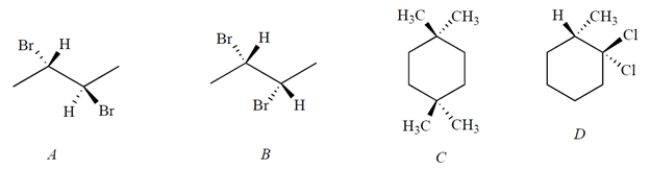
A) compound A
B) compound B
C) compound C
D) compound D
E) none of these compounds

A) compound A
B) compound B
C) compound C
D) compound D
E) none of these compounds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
7
Four stereoisomers of the naturally occurring amino acid isoleucine are given. L-Isoleucine is the stereoisomer with the 2S,3S configuration. Identify this stereoisomer.
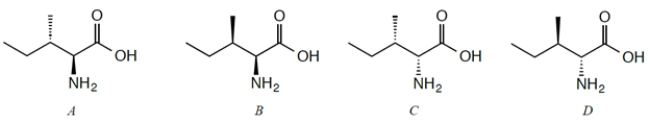
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
8
Four stereoisomers of the naturally occurring amino acid isoleucine are given. L-Isoleucine is the stereoisomer with the 2S,3S configuration. Which configurations are diastereomers of L-Isoleucine?
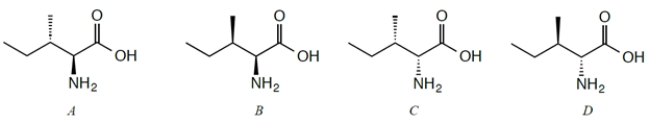
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
9
The structure of the beta-blocker drug Nadolol is given. For the two non-racemic stereocenters, C2 and C3 (labeled), please give the absolute configuration of each.
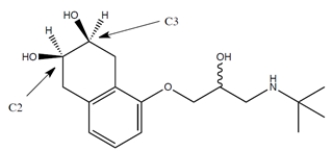


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
10
Select the true statements.
A) If a compound with a single asymmetric carbon has the S configuration, it has levorotatory optical activity.
B) Any compound with one asymmetric carbon is chiral.
C) Meso compounds are chiral.
D) For any reaction that does not affect the bonds to an asymmetric carbon, if the configuration of this carbon is R, the products must also have the R configuration at this carbon.
E) A compound X with four asymmetric carbons is the enantiomer of a compound Y. Compound X must then have a diastereomer Z.
A) If a compound with a single asymmetric carbon has the S configuration, it has levorotatory optical activity.
B) Any compound with one asymmetric carbon is chiral.
C) Meso compounds are chiral.
D) For any reaction that does not affect the bonds to an asymmetric carbon, if the configuration of this carbon is R, the products must also have the R configuration at this carbon.
E) A compound X with four asymmetric carbons is the enantiomer of a compound Y. Compound X must then have a diastereomer Z.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
11
Imagine a hypothetical reaction to make a chiral molecule that gives the desired product (the R enantiomer, which has a specific rotation of −95o mL g−1 dm−1) in 68% EE. What percentage of the product is R, and what percentage is S? (Show your work.)
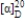
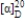

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
12
Imagine a hypothetical reaction to make a chiral molecule that gives the desired product (the R enantiomer, which has a specific rotation of −55o mL g−1 dm−1) in 40% EE. What is the specific rotation of the S enantiomer?
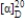

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
13
Imagine a hypothetical reaction to make a chiral molecule that gives the desired product (the R enantiomer, which has a specific rotation of −80ᵒ mL g−1 dm−1) in 75% EE. Assuming a path length of 1 dm, what would be the observed optical rotation of a solution of 1 g of this 75% EE product mixture dissolved in 1 mL of solvent? (Show your work.)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
14
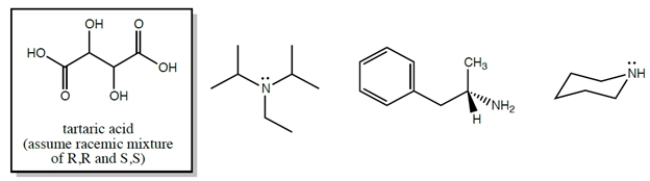
Circle any of the structures that could be used for enantiomeric resolution of a racemic mixture of the (R,R) and (S,S) enantiomers of tartaric acid.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
15
Enantiomerically pure (S)-2-bromobutane has a specific optical rotation = +23.1ᵒ. A sample of 2-bromobutane containing some of each enantiomer has a specific rotation of −9.2ᵒ.
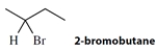 a. The enantiomeric excess of the dominant enantiomer in the mixture is ________________.
a. The enantiomeric excess of the dominant enantiomer in the mixture is ________________.
b. The percentage of the R enantiomer in the mixture is ________________, and the S enantiomer is ________________.
 a. The enantiomeric excess of the dominant enantiomer in the mixture is ________________.
a. The enantiomeric excess of the dominant enantiomer in the mixture is ________________.b. The percentage of the R enantiomer in the mixture is ________________, and the S enantiomer is ________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
16
Select the two chiral compounds in this set.
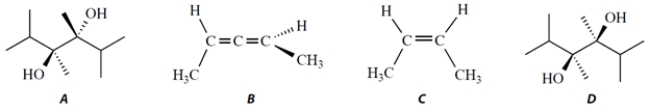
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
17
Linezolid is an antibiotic used against gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It is sold by Pfizer under the trade name Zyvox® as a single enantiomer.
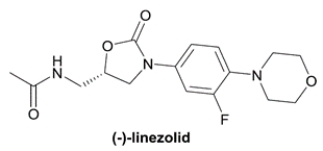 a. Mark the asymmetric center with an asterisk (*) in the structure of (−)-linezolid. The Cahn-Ingold-Prelog configuration of the asymmetric center in (−)-linezolid is ___________.
a. Mark the asymmetric center with an asterisk (*) in the structure of (−)-linezolid. The Cahn-Ingold-Prelog configuration of the asymmetric center in (−)-linezolid is ___________.
b. Assuming that linezolid is synthesized as a racemic mixture, name a method that chemists at Pfizer might be able to utilize to isolate the two enantiomers from racemic linezolid.
 a. Mark the asymmetric center with an asterisk (*) in the structure of (−)-linezolid. The Cahn-Ingold-Prelog configuration of the asymmetric center in (−)-linezolid is ___________.
a. Mark the asymmetric center with an asterisk (*) in the structure of (−)-linezolid. The Cahn-Ingold-Prelog configuration of the asymmetric center in (−)-linezolid is ___________.b. Assuming that linezolid is synthesized as a racemic mixture, name a method that chemists at Pfizer might be able to utilize to isolate the two enantiomers from racemic linezolid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
18
For the racemic acid:
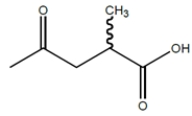 Which structures could be effective as resolving agents for separation of the enantiomers for the racemic acid?
Which structures could be effective as resolving agents for separation of the enantiomers for the racemic acid?
A)
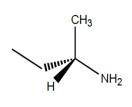
B)
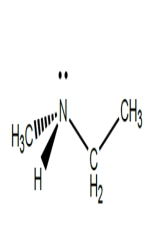
C)
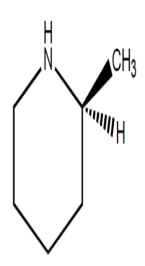
D) (2S, 3S)-tartaric acid
 Which structures could be effective as resolving agents for separation of the enantiomers for the racemic acid?
Which structures could be effective as resolving agents for separation of the enantiomers for the racemic acid?A)

B)

C)

D) (2S, 3S)-tartaric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
19
Identify the stereocenters in the structure.
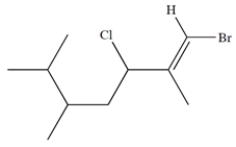


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
20
The structures of two of the stereoisomers of tartaric acid are shown.
 a. Give the structures of all other stereoisomer(s) of tartaric acid on the templates below. You may or may not need all the templates. Put a large "X" through the ones that you do not need, if any.
a. Give the structures of all other stereoisomer(s) of tartaric acid on the templates below. You may or may not need all the templates. Put a large "X" through the ones that you do not need, if any.
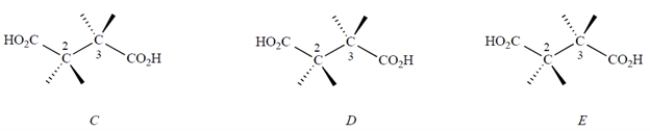 b. What is the stereochemical relationship of each stereoisomer you drew to each of the two stereoisomers shown above?
b. What is the stereochemical relationship of each stereoisomer you drew to each of the two stereoisomers shown above?
c. What, if anything, can you say about the optical activity of each of the stereoisomers that you drew?
 a. Give the structures of all other stereoisomer(s) of tartaric acid on the templates below. You may or may not need all the templates. Put a large "X" through the ones that you do not need, if any.
a. Give the structures of all other stereoisomer(s) of tartaric acid on the templates below. You may or may not need all the templates. Put a large "X" through the ones that you do not need, if any. b. What is the stereochemical relationship of each stereoisomer you drew to each of the two stereoisomers shown above?
b. What is the stereochemical relationship of each stereoisomer you drew to each of the two stereoisomers shown above?c. What, if anything, can you say about the optical activity of each of the stereoisomers that you drew?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
21
The structure of atorvastatin, an important drug used for lowering cholesterol, is shown. (This is sold under the trade name Lipitor with about $13 billion in annual sales.)
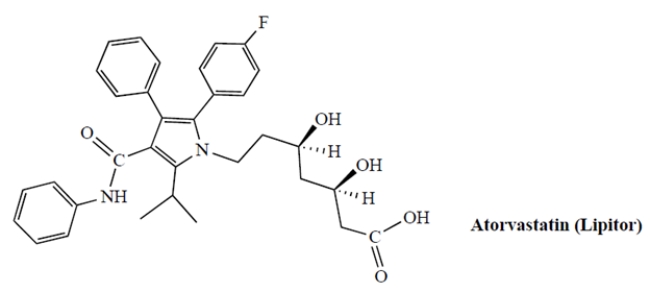 a. Circle the two asymmetric carbons of atorvastatin and, next to each, give its absolute configuration (R or S).
a. Circle the two asymmetric carbons of atorvastatin and, next to each, give its absolute configuration (R or S).
b. What, if anything, can you say about the optical activity of atorvastatin?
 a. Circle the two asymmetric carbons of atorvastatin and, next to each, give its absolute configuration (R or S).
a. Circle the two asymmetric carbons of atorvastatin and, next to each, give its absolute configuration (R or S).b. What, if anything, can you say about the optical activity of atorvastatin?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the stereochemistry of the stereoisomeric salt that has the same melting point as this salt? (The numbers refer to the carbons whose stereochemistry is given below.)
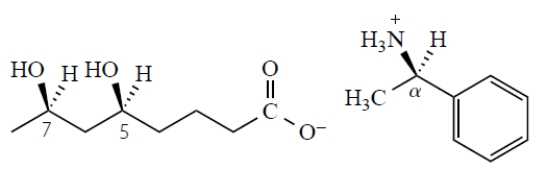
A) 5S,7S, R
B) 5S,7S, S
C) 5S,7R, R
D) 5R,7S, S
E) 5R,7S, Rf.
5R,7R, S

A) 5S,7S, R
B) 5S,7S, S
C) 5S,7R, R
D) 5R,7S, S
E) 5R,7S, Rf.
5R,7R, S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
23
A mixture of enantiomers of 5-penten-2-ol has an apparent specific rotation of +9.89º mL g−1 dm−1 at temperature = 25 ºC in ether solvent. The sample is known to have an optical purity (= enantiomeric excess) of 67%.
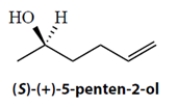 a. What is the specific rotation of the pure S enantiomer under the same conditions? Show your work.
a. What is the specific rotation of the pure S enantiomer under the same conditions? Show your work.
b. What percentage of each enantiomer is present in the mixture? Show your work.
%S enantiomer:_________________; %R enantiomer: ______________
 a. What is the specific rotation of the pure S enantiomer under the same conditions? Show your work.
a. What is the specific rotation of the pure S enantiomer under the same conditions? Show your work.b. What percentage of each enantiomer is present in the mixture? Show your work.
%S enantiomer:_________________; %R enantiomer: ______________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
24
The structure of pseudoephedrine, a common decongestant, is shown below. Compare the structure of pseudoephedrine with the Newman projection of compound X. The relationship between pseudoephedrine and compound X is
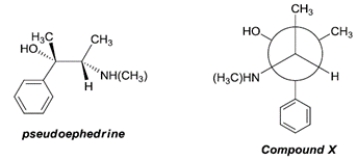
A) identical
B) enantiomers
C) diastereomers
D) constitutional isomers

A) identical
B) enantiomers
C) diastereomers
D) constitutional isomers

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
25
Vasotec, a drug prescribed to treat hypertension, is a salt of enalapril cation and maleate anion.
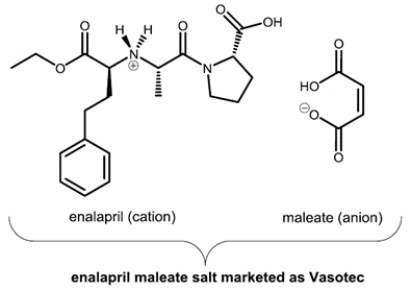 a. What is the number of asymmetric centers in Vasotec?
a. What is the number of asymmetric centers in Vasotec?
b. What is the number of stereocenters in Vasotec?
c. There are multiple asymmetric centers in Vasotec. Mark an asymmetric center in the structure of Vasotec above with an asterisk (*). What is the configuration of the asymmetric center you have identified (R/S)?
d. What is the configuration of the double bond in maleate anion (E/Z)?
 a. What is the number of asymmetric centers in Vasotec?
a. What is the number of asymmetric centers in Vasotec?b. What is the number of stereocenters in Vasotec?
c. There are multiple asymmetric centers in Vasotec. Mark an asymmetric center in the structure of Vasotec above with an asterisk (*). What is the configuration of the asymmetric center you have identified (R/S)?
d. What is the configuration of the double bond in maleate anion (E/Z)?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck








