Deck 17: Allylic and Benzylic Reactivity
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/25
العب
ملء الشاشة (f)
Deck 17: Allylic and Benzylic Reactivity
1
The structure of geraniol is shown. Label the allylic carbons with an asterisk (*).
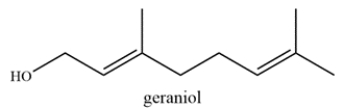

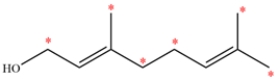
An allylic carbon is a carbon directly adjacent to a double bond. Each alkene is trisubstituted and each alkyl substituent is an allylic carbon, so there are six allylic carbons labeled.

2
Consider the structure of eugenol, which is commonly found in essential oils and is used in perfumes.
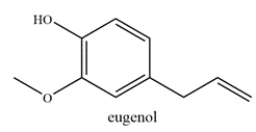 Which statement is true?
Which statement is true?
A) Eugenol contains an allylic carbon and no benzylic carbons.
B) Eugenol contains a benzylic carbon and no allylic carbons.
C) Eugenol contains both an allylic and a benzylic carbon.
D) Eugenol does not contain an allylic nor a benzylic carbon.
 Which statement is true?
Which statement is true?A) Eugenol contains an allylic carbon and no benzylic carbons.
B) Eugenol contains a benzylic carbon and no allylic carbons.
C) Eugenol contains both an allylic and a benzylic carbon.
D) Eugenol does not contain an allylic nor a benzylic carbon.
C
3
In each set of reactions, circle the one that occurs faster.
a.
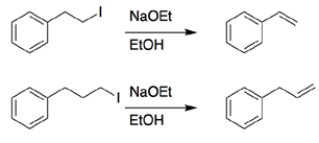 b.
b.
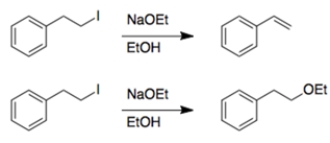
a.
 b.
b. 
a. Both reactions are eliminations. In the first reaction, the beta hydrogen removed during elimination is allylic, so it will be easier to remove, and the reaction will be faster.
b. Both reactions have the same starting material and conditions, so you are comparing substitution versus elimination. Again, the beta proton eliminated in the first reaction is allylic, so it will be easier to remove, and the reaction will be faster.
b. Both reactions have the same starting material and conditions, so you are comparing substitution versus elimination. Again, the beta proton eliminated in the first reaction is allylic, so it will be easier to remove, and the reaction will be faster.
4
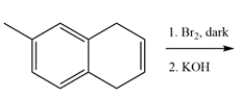
Determine the major organic product for the reaction.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
5
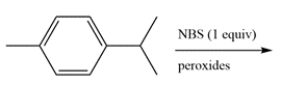
Determine the major organic product for the reaction. The product should have a molecular formula of C10H13Br.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
6
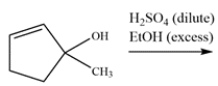
The alcohol reacts with excess ethanol and acid to give two different products, both of which show an alkene stretch in their respective IR spectra. Draw a mechanism for the reaction and show why two products can be formed. Identify both products.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of these compounds is the most acidic?
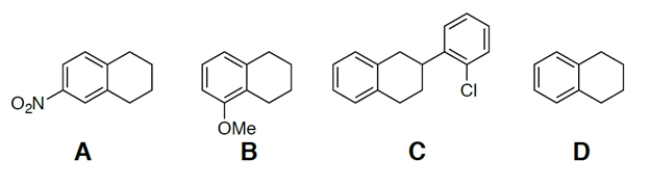
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
8
Determine the major organic product for the reaction.
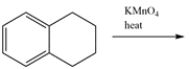


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
9
Determine the major organic product for the reaction.
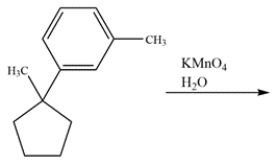


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
10
Determine the major organic product for the reaction.
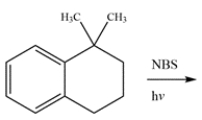


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
11
Propose a multistep synthesis for the compound from toluene. You may assume that ortho and para isomers can be separated as necessary. Reaction mechanisms are not necessary.
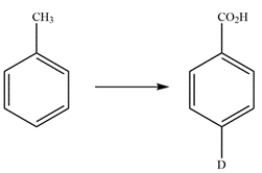


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
12
Rationalize these two observations through analysis of intermediates.
1. Solvolysis of bromocyclopentene in methanol is much faster than is solvolysis of bromocyclopentane.
2. Solvolysis of bromocyclopentadiene is slower than solvolysis of bromocyclopentane.
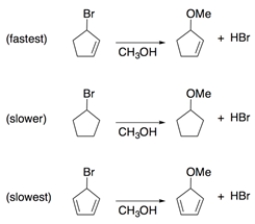
1. Solvolysis of bromocyclopentene in methanol is much faster than is solvolysis of bromocyclopentane.
2. Solvolysis of bromocyclopentadiene is slower than solvolysis of bromocyclopentane.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which substrate will undergo the reaction the fastest?
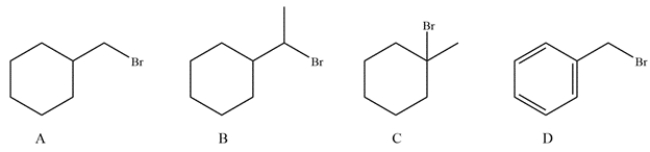
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
14
These compounds have very different acidities. State which is the most acidic, which is the least acidic, which is in the middle, and why.
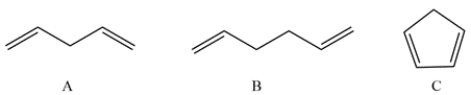


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
15
Draw the major organic products for the reactions.
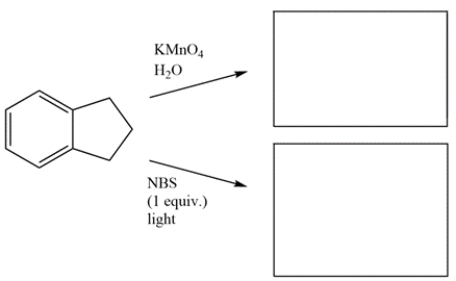


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
16
Draw the major organic product for the reaction.
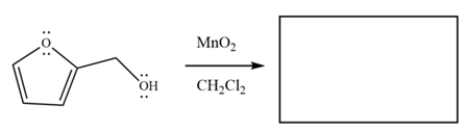


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
17
Draw the major organic product for the reaction.
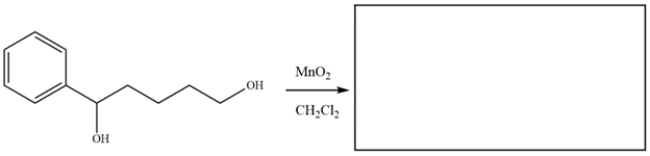


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
18
The compound was synthesized from MnO2 oxidation. Deduce the structure of the starting material.
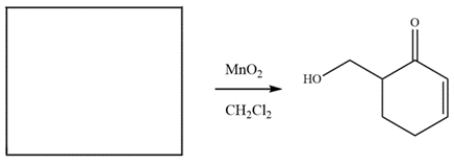


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
19
Consider these alcohols and explain whether oxidation with pyridinium chlorochromate (PCC) and MnO2 would give the same product, different products or no reaction.
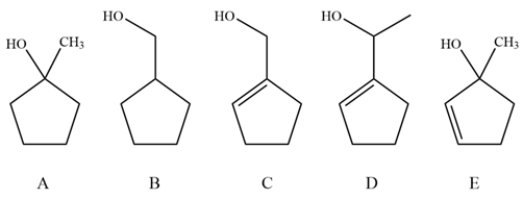


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
20
Carvone is a terpenoid that is found in essential oils and used for aromatherapy. Highlight the isoprene units in carvone and classify the terpene based on the number of carbons (ex. monoterpene, etc.)
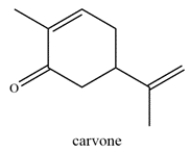


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
21
Humulene is a terpene that is responsible for the hops aroma in beer. Highlight the isoprene units in humulene and classify the terpene based on the number of carbons (ex. monoterpene, etc.)
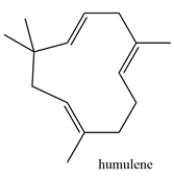


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
22
Outline a synthesis of 3-bromobenzaldehyde from toluene.



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
23
Deduce the structure of the starting material.
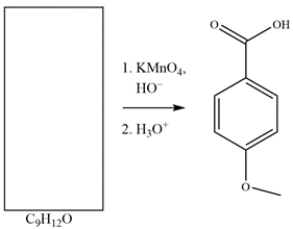


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
24
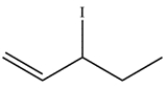
A bottle labeled 3-iodo-1-pentene (structure below) was stored in the back of the laboratory refrigerator. Several weeks later, a chemist analyzed the compound and found that it was contaminated with a different molecule with the same molecular formula. Determine the structure of the isomer and draw a curved-arrow mechanism to explain how it was formed.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
25
A student treated the Grignard reagent with D2O and was surprised to isolate two isomeric products. Identify the two products formed and explain why both were formed.



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck








