Deck 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/25
العب
ملء الشاشة (f)
Deck 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes
1
Complete the reaction by giving the missing product.
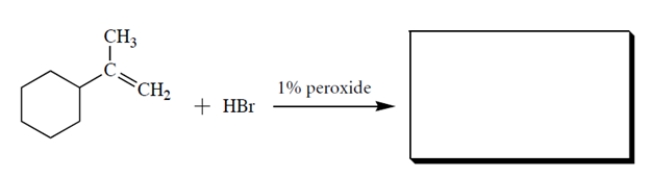

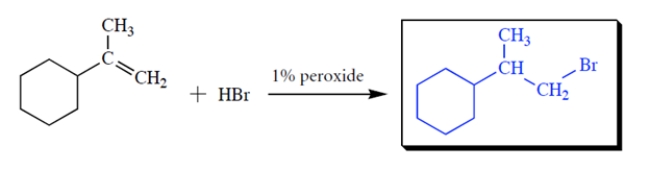
2
Give the structure of the organic free-radical intermediate formed in the propagation steps in the reaction of the alkene with HBr in the presence of peroxides.
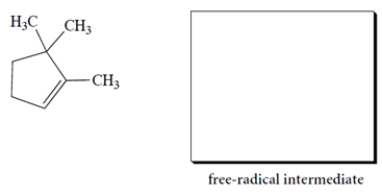

The bromine will add to the less substituted alkene carbon and generate the more stable radical, the tertiary radical. The structure of the free-radical intermediate is
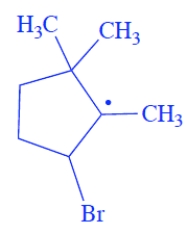

3
Give the product A that results when HBr undergoes peroxide-mediated addition to 1-methylcyclopentene. Also give the structure of the free-radical intermediate B (other than Br.) in the propagation steps of this reaction.
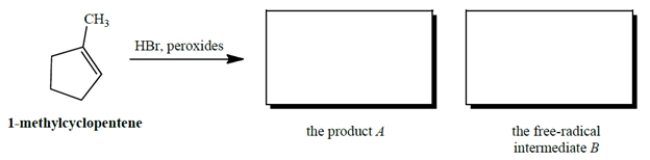

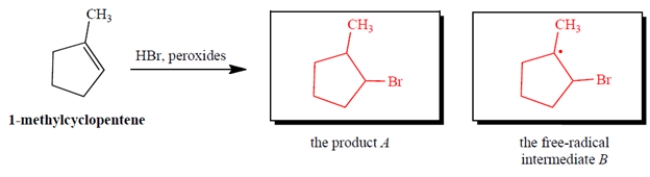
4
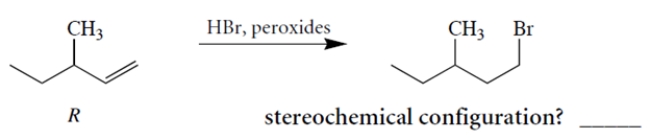
Treatment of (+)-(R)-3-methyl-1-pentene with HBr and peroxides yields (-)-1-bromo-3-methyl pentane. Determine the stereochemical configuration of the product.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
5
Thiols (R-S-H) undergo addition to alkenes but only in the presence of peroxides. This equation is an example.
Which one of these is a reactive intermediate in this reaction?
(a)
(b)
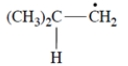
(c)
(d)
(e)
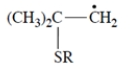
(f)
A) compound (a)
B) compound (b)
C) compound (c)
D) compound (d)
E) compound (e)f.
compound (f)
Which one of these is a reactive intermediate in this reaction?
(a)
(b)
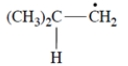
(c)
(d)
(e)

(f)
A) compound (a)
B) compound (b)
C) compound (c)
D) compound (d)
E) compound (e)f.
compound (f)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
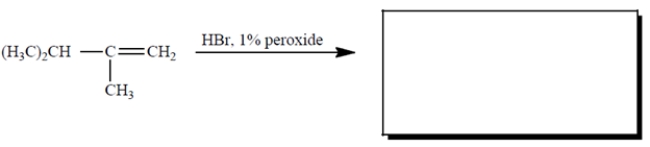
6
Complete the reaction by giving the missing product.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
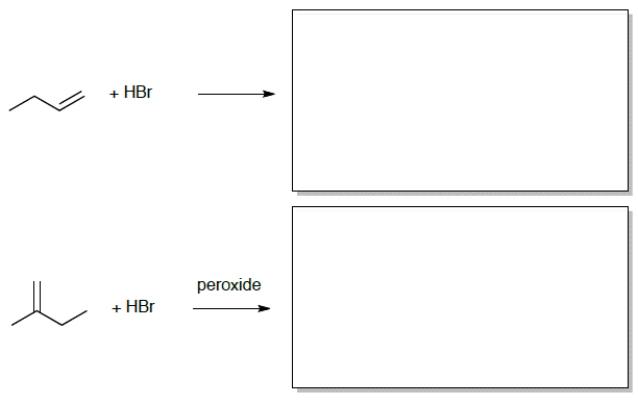
7
Draw the correct product for each of the reactions:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
8
Complete the reaction by giving the major organic product(s).
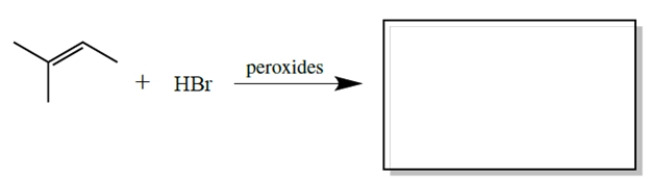


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
9
Consider this addition polymer, called poly(methyl methacrylate). What is the structure of the monomer from which this polymer is formed by free-radical alkene polymerization?
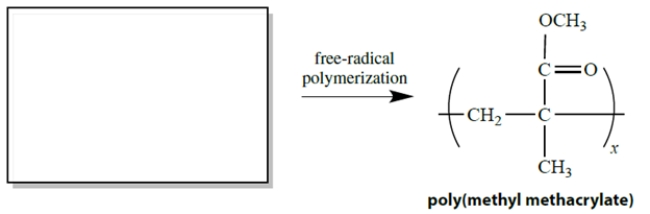


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
10
Complete the reactions by giving the structure of the missing organic products.
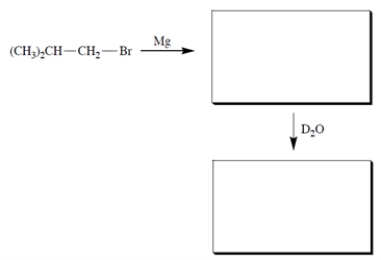


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
11
Complete the reaction by giving the structures of all products.
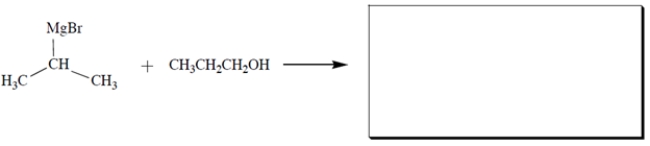


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
12
Provide the structures of the missing compounds.
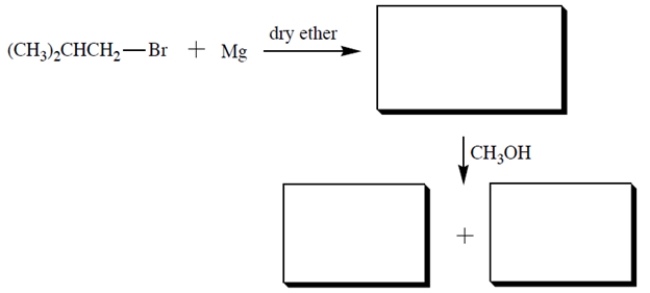


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of these compounds is capable of spontaneously providing a free-radical RO• that can initiate free-radical chain reactions?
A) HO-OH
B) (CH3)3O-OC(CH3)3
C) (CH3)3-O-C(CH3)3
D) (CH3)3O-OH
E) All can serve as initiators.
A) HO-OH
B) (CH3)3O-OC(CH3)3
C) (CH3)3-O-C(CH3)3
D) (CH3)3O-OH
E) All can serve as initiators.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
14
Free-radical polymerization of ethylene proceeds with the following propagation steps.
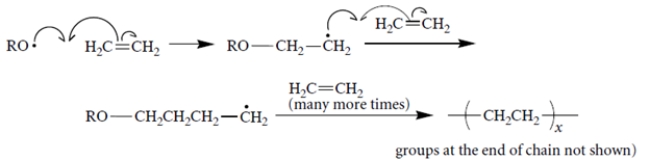 It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
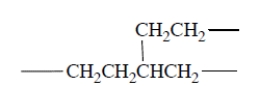 (a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(b) It is possible (by a completely different method) to form polyethylene with unbranched chains. Which do you think would have higher density, branched-chain polyethylene or polyethylene with unbranched chains? Explain briefly.
 It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
It turns out that polyethylene made by free-radical polymerization contains some branches, for example,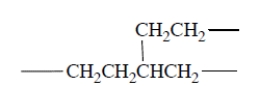 (a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.(b) It is possible (by a completely different method) to form polyethylene with unbranched chains. Which do you think would have higher density, branched-chain polyethylene or polyethylene with unbranched chains? Explain briefly.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
15
Devise a synthesis to achieve the transformation.
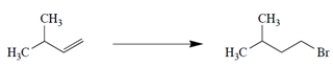


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
16
1-Methyl-1-vinyl cyclopentane undergoes addition of HBr under two different conditions as shown in the two pathways. A mixture of products is obtained in Pathway A, whereas a single product is obtained in pathway

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
17
A free-radical addition of a certain compound X-Y to an alkene (R = alkyl) results in the conversion:
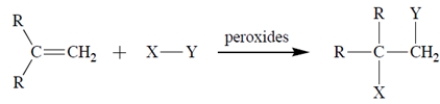 The first propagation step in the mechanism is
The first propagation step in the mechanism is
 Therefore, what is the second propagation step in the mechanism?
Therefore, what is the second propagation step in the mechanism?
A)
B)
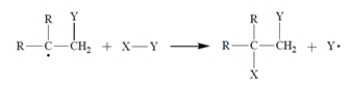
C)
D) Both (b) and (c) are propagation steps.
E) Both (a) and (b) are propagation steps.
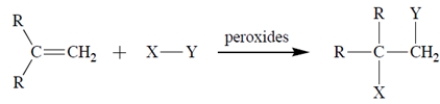 The first propagation step in the mechanism is
The first propagation step in the mechanism is Therefore, what is the second propagation step in the mechanism?
Therefore, what is the second propagation step in the mechanism?A)

B)
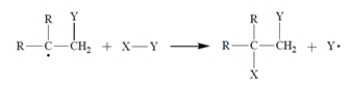
C)
D) Both (b) and (c) are propagation steps.
E) Both (a) and (b) are propagation steps.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
18
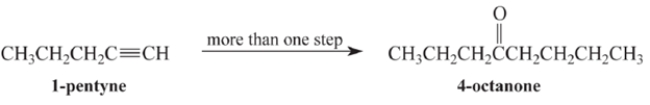
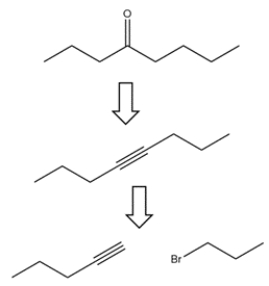
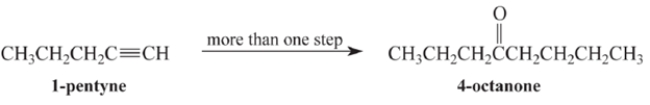
Outline a multi-step synthesis of 4-octanone from 1-pentyne and any other reagents. (Show the reagents required for each step, and the product of each step. Don't show the mechanism.)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
19
Complete the reactions by giving the structure of the missing organic products.
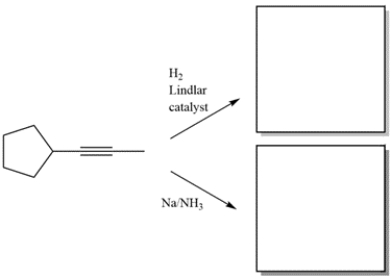


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
20
Complete the reactions by giving the structure of the missing organic products.
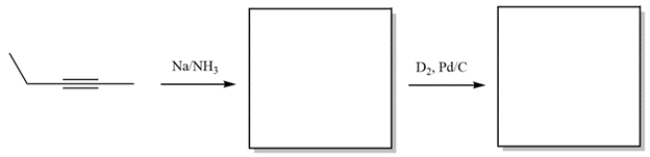


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
21
Identify the stereochemistry of the polymers.
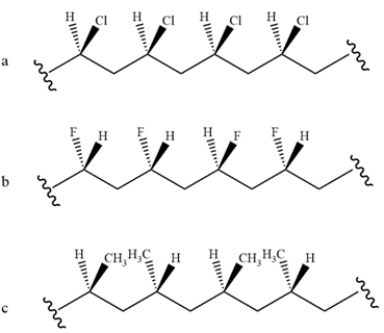


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
22
Predict the major organic product of the reaction:
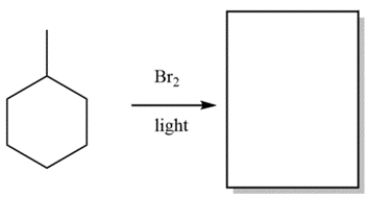


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
23
Outline a synthesis for the alkyne using acetylene and any reagents three carbons or less.
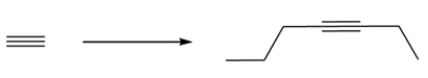


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
24
Outline a synthesis of this product.
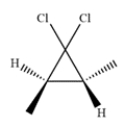


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck
25
Provide the major organic product for the reaction.
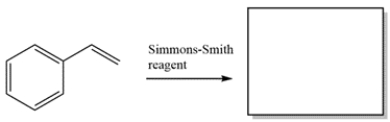


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 25 في هذه المجموعة.
فتح الحزمة
k this deck








