Deck 1: Chemical Bonding and Chemical Structure
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/26
العب
ملء الشاشة (f)
Deck 1: Chemical Bonding and Chemical Structure
1
Which compound has the largest dipole moment?
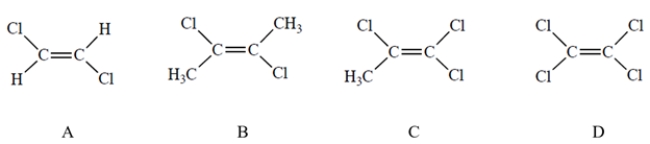
A) Compound A
B) Compound B
C) Compound C
D) Compound D

A) Compound A
B) Compound B
C) Compound C
D) Compound D
C
2
Which atomic orbital has two nodes?
A) 2p
B) 3p
C) 4s
D) 1s
E) none of these
A) 2p
B) 3p
C) 4s
D) 1s
E) none of these
B
3
Which statement is true about molecular orbitals of any given molecule?
A) A bonding molecular orbital has higher energy and fewer nodes than an antibonding molecular orbital.
B) A bonding molecular orbital can have the same energy as an antibonding orbital.
C) Any antibonding orbital is never populated with electrons.
D) Any bonding molecular orbital always contains two electrons.
E) None of these is true.
A) A bonding molecular orbital has higher energy and fewer nodes than an antibonding molecular orbital.
B) A bonding molecular orbital can have the same energy as an antibonding orbital.
C) Any antibonding orbital is never populated with electrons.
D) Any bonding molecular orbital always contains two electrons.
E) None of these is true.
E
4
Which one of the following statements about the hydrogen molecule cation, H2+ , is true?
A) This cation is unstable because it does not have two electrons in a bonding molecular orbital.
B) The H-H bond in this cation is stronger than the H-H bond in the dihydrogen molecule H2.
C) This cation has one electron in the bonding molecular orbital and one electron in an antibonding molecular orbital.
D) This cation is stable relative to a hydrogen atom and a proton (H+).
A) This cation is unstable because it does not have two electrons in a bonding molecular orbital.
B) The H-H bond in this cation is stronger than the H-H bond in the dihydrogen molecule H2.
C) This cation has one electron in the bonding molecular orbital and one electron in an antibonding molecular orbital.
D) This cation is stable relative to a hydrogen atom and a proton (H+).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many valence electrons does aluminum (Al, atomic number = 13) have?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the formal charge on carbon in this structure? All unshared electrons are shown.
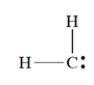


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
7
Complete the structure for the cyanate ion by adding unshared electron pairs and formal charges. Every atom has an octet, and the overall charge on the ion is −1.
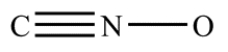
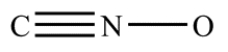

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the C-N-O bond angle in the cyanate ion?
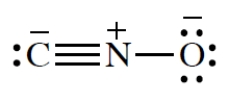
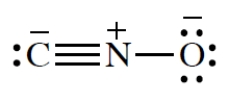

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
9
The occupied valence orbitals of the chlorine atom are
A) 2s and 2p.
B) 3s.
C) 3s and 3p.
D) 3s and 3p and 3d.
E) 4s and 4p.
A) 2s and 2p.
B) 3s.
C) 3s and 3p.
D) 3s and 3p and 3d.
E) 4s and 4p.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
10
Identify the true statement(s) about molecular orbitals (MOs).
A) Antibonding MOs always have more nodes than bonding orbitals.
B) Antibonding MOs always have higher energy than bonding MOs.
C) All bonding MOs have zero nodes.
A) Antibonding MOs always have more nodes than bonding orbitals.
B) Antibonding MOs always have higher energy than bonding MOs.
C) All bonding MOs have zero nodes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
11
Consider this compound:
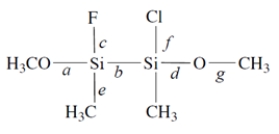 1. Of the labeled bonds, the longest bond is ________________.
1. Of the labeled bonds, the longest bond is ________________.
2. The most polar bond is ________________.
3. The bonding geometry at the silicon atoms is ________________.
4. The hybridization of the silicon atoms is ________________.
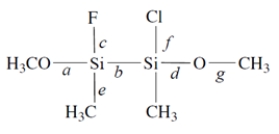 1. Of the labeled bonds, the longest bond is ________________.
1. Of the labeled bonds, the longest bond is ________________.2. The most polar bond is ________________.
3. The bonding geometry at the silicon atoms is ________________.
4. The hybridization of the silicon atoms is ________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
12
The atomic orbital that cannot exist is
A) 1s.
B) 1p.
C) 2s.
D) 2p.
E) 3df.
All can exist.
A) 1s.
B) 1p.
C) 2s.
D) 2p.
E) 3df.
All can exist.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
13
The lactate ion is a resonance hybrid:
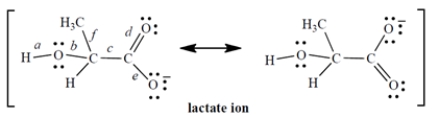
1. Considering only the labeled bonds, the longest carbon-oxygen bond in the lactate ion is ________________.
2. Considering only the labeled bonds, the two bonds that have the same length are ________________ and ________________.

1. Considering only the labeled bonds, the longest carbon-oxygen bond in the lactate ion is ________________.
2. Considering only the labeled bonds, the two bonds that have the same length are ________________ and ________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
14
According to molecular orbital theory, which one of these species does not exist?
A)
B)
C)
D)
E) CH4
A)
B)
C)
D)
E) CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
15
Select the two statements that are true.
A) All polar molecules have a significant dipole moment.
B) All molecules containing polar bonds are polar molecules.
C) The polarity of a bond is accurately indicated in every case by the formal charges of the atoms involved in the bond.
D) All polar molecules contain one or more polar bonds.
A) All polar molecules have a significant dipole moment.
B) All molecules containing polar bonds are polar molecules.
C) The polarity of a bond is accurately indicated in every case by the formal charges of the atoms involved in the bond.
D) All polar molecules contain one or more polar bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
16
Give the formal charge on the phosphorus atom in this structure. (Phosphorus is in group 5A directly under nitrogen in the periodic table.)
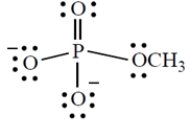


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
17
Of the bonds marked, which bond is the shortest?
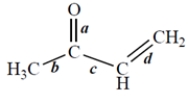


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
18
Given that the acetate anion has the these equally important resonance structures, what is the bond order of each carbon-oxygen bond?
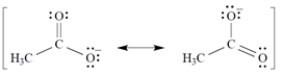
A) The bond order of all carbon-oxygen bonds is ⅓.
B) The bond order of all carbon-oxygen bonds is ½.
C) The bond order of all carbon-oxygen bonds is 1.0.
D) The bond order of all carbon-oxygen bonds is 1.5.
E) The bond order of one carbon-oxygen bond is 1.0, and the bond order of the other carbon-oxygen is ½.f.
The bond order of all carbon-oxygen bonds is 2.0.

A) The bond order of all carbon-oxygen bonds is ⅓.
B) The bond order of all carbon-oxygen bonds is ½.
C) The bond order of all carbon-oxygen bonds is 1.0.
D) The bond order of all carbon-oxygen bonds is 1.5.
E) The bond order of one carbon-oxygen bond is 1.0, and the bond order of the other carbon-oxygen is ½.f.
The bond order of all carbon-oxygen bonds is 2.0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
19
Add valence electrons to the structures so that the formal charge is properly accounted for. Assume that all atoms have no more electrons than allowed by the octet rule. Make your "electron dots" bold enough to be unambiguous.
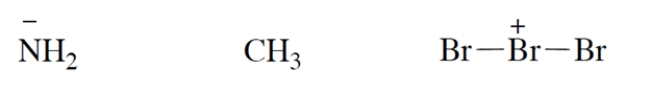
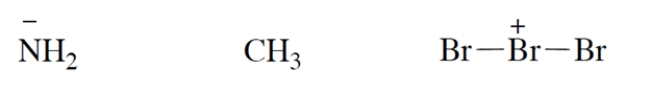

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the geometry of the borohydride ion, -BH4?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
21
Complete the electron configuration diagram below for the element boron (B, atomic number = 5), showing 1s, 2s, 2px, 2py, and 2pz orbitals, their relative energies, and their electron populations indicated by "spin arrows" ↑ and ↓.
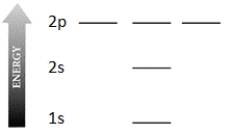
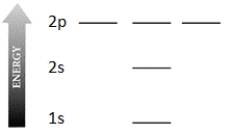

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
22
Assume that the structure of BF3 (boron trifluoride, or trifluoroborane) is correctly predicted by the VSEPR rules.
1. What is the predicted structure? (Show all unpaired valence electrons.)
2. The B-F bond is exceptionally strong. This suggests that it has some double-bond character. Draw resonance structures for BF3 that show that the three B-F bonds share some double-bond character. Be sure to show formal charges and all unshared electrons.
3. What is the polarity of the B-F bond? (In which direction is the bond dipole?) How do you know?
1. What is the predicted structure? (Show all unpaired valence electrons.)
2. The B-F bond is exceptionally strong. This suggests that it has some double-bond character. Draw resonance structures for BF3 that show that the three B-F bonds share some double-bond character. Be sure to show formal charges and all unshared electrons.
3. What is the polarity of the B-F bond? (In which direction is the bond dipole?) How do you know?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
23
Consider this structure:
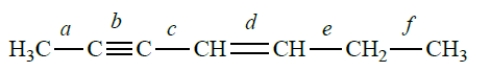 Which bond represents an sp-sp2 carbon-carbon bond?
Which bond represents an sp-sp2 carbon-carbon bond?
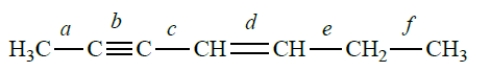 Which bond represents an sp-sp2 carbon-carbon bond?
Which bond represents an sp-sp2 carbon-carbon bond?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
24
Consider this structure:
The bond angle between bonds c and d is approximately
A) 109.5°.
B) 120°.
C) 60°.
D) 180°
The bond angle between bonds c and d is approximately
A) 109.5°.
B) 120°.
C) 60°.
D) 180°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
25
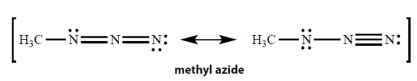
In the resonance structures for methyl azide, all unshared electron pairs are indicated. Complete the structures by adding the missing formal charges.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck
26
Consider this structure:
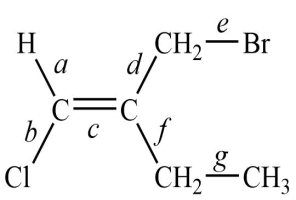 1. The longest bond is ________________.
1. The longest bond is ________________.
2. The shortest of the carbon-carbon bonds is ________________.
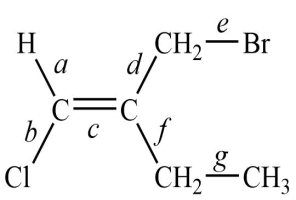 1. The longest bond is ________________.
1. The longest bond is ________________.2. The shortest of the carbon-carbon bonds is ________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 26 في هذه المجموعة.
فتح الحزمة
k this deck








