Deck 5: Solutions and Concentrations
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/19
العب
ملء الشاشة (f)
Deck 5: Solutions and Concentrations
1
A solution in which the solvent is water is referred to as
A) aqueous.
B) dissolved.
C) liquid.
D) soluble.
A) aqueous.
B) dissolved.
C) liquid.
D) soluble.
aqueous.
2
A solution is composed of 1% sodium chloride and 0.5% potassium chloride in distilled water.Which substance(s)would be considered the solute?
A) Potassium chloride
B) Sodium chloride
C) Potassium chloride and sodium chloride
D) Water
A) Potassium chloride
B) Sodium chloride
C) Potassium chloride and sodium chloride
D) Water
Potassium chloride and sodium chloride
3
Which of the following best defines the term "solution"?
A) A combination of two or more different chemical elements in which the atoms of the different elements are held together by chemical bonds
B) A heterogeneous combination of two or more substances where the properties of each individual substance are absorbed by the other(s)
C) A homogeneous mixture of two or more substances that do not chemically react with each other
D) A mixture of two or more substances that results in a change in the molecular composition of each substance
A) A combination of two or more different chemical elements in which the atoms of the different elements are held together by chemical bonds
B) A heterogeneous combination of two or more substances where the properties of each individual substance are absorbed by the other(s)
C) A homogeneous mixture of two or more substances that do not chemically react with each other
D) A mixture of two or more substances that results in a change in the molecular composition of each substance
A homogeneous mixture of two or more substances that do not chemically react with each other
4
What are the units on a pH value?
A) g/mL
B) M/L
C) mol/L
D) None of the above
A) g/mL
B) M/L
C) mol/L
D) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the specific gravity of a solution?
A) The ratio of the concentration of a solute to the concentration of the solvent at 37°C
B) The ratio of the concentration of a solute to the density of water at 4°C
C) The ratio of the density of a solute to the density of the solvent at 37°C
D) The ratio of the density of a solution to the density of water at 4°C
A) The ratio of the concentration of a solute to the concentration of the solvent at 37°C
B) The ratio of the concentration of a solute to the density of water at 4°C
C) The ratio of the density of a solute to the density of the solvent at 37°C
D) The ratio of the density of a solution to the density of water at 4°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
6
A solution of sodium chloride (NaCl)is calculated to be 0.5 M at 37°C.The solution was placed in an 18°C refrigerator for 24 hours and the molarity was recalculated.What would you expect the molarity of the solution to be?
A) 0.5 M
B) Greater than 0.5 M
C) Less than 0.5 M
D) It cannot be determined from the information given.
A) 0.5 M
B) Greater than 0.5 M
C) Less than 0.5 M
D) It cannot be determined from the information given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
7
When a concentration is expressed in terms of molarity,what does it tell us about the solution?
A) The number of moles of a solute present in 1 liter of solution
B) The number of moles of a solute present in 1,000 grams of solution
C) The number of osmoles of a solute present in 1 liter of solution
D) The number of osmoles of a solute present in 1,000 grams of solution
A) The number of moles of a solute present in 1 liter of solution
B) The number of moles of a solute present in 1,000 grams of solution
C) The number of osmoles of a solute present in 1 liter of solution
D) The number of osmoles of a solute present in 1,000 grams of solution

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
8
A solution has pH 7.Another solute is added to the solution and the pH changes to 6.What does this tell us about the concentration of hydrogen ions in the solution?
A) The hydrogen ion concentration decreased by a factor of 10
B) The hydrogen ion concentration decreased by a factor of 100
C) The hydrogen ion concentration increased by a factor of 10
D) The hydrogen ion concentration increased by a factor of 100
A) The hydrogen ion concentration decreased by a factor of 10
B) The hydrogen ion concentration decreased by a factor of 100
C) The hydrogen ion concentration increased by a factor of 10
D) The hydrogen ion concentration increased by a factor of 100

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the rule for determining an equivalent for calculating the normality of a base or salt solution?
A) An equivalent is the number of hydrogen atoms present in the base or salt.
B) An equivalent is the number of hydrogen ions with which it can theoretically combine.
C) An equivalent is the total number of hydroxide (OH⁻) groups divided by the number of hydrogen atoms present.
D) An equivalent is the total number of positively charged ions present in the molecule.
A) An equivalent is the number of hydrogen atoms present in the base or salt.
B) An equivalent is the number of hydrogen ions with which it can theoretically combine.
C) An equivalent is the total number of hydroxide (OH⁻) groups divided by the number of hydrogen atoms present.
D) An equivalent is the total number of positively charged ions present in the molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
10
What does the term "equivalent weight" mean?
A) The amount of a solute that is equivalent in mass to 1 mole of the intended solvent
B) The amount of a substance that contains, theoretically combines with, or theoretically replaces 1 mole of hydrogen atoms
C) The molality of a solute that contains the same number of atoms as 100 grams of solvent
D) The molarity of a solute that is necessary to completely saturate 100 mL of solvent
A) The amount of a solute that is equivalent in mass to 1 mole of the intended solvent
B) The amount of a substance that contains, theoretically combines with, or theoretically replaces 1 mole of hydrogen atoms
C) The molality of a solute that contains the same number of atoms as 100 grams of solvent
D) The molarity of a solute that is necessary to completely saturate 100 mL of solvent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the risk of reporting an incorrect concentration for a patient sample?
A) Administration of an inappropriate drug
B) Misdiagnosis of an illness
C) Both of the above
D) Neither of the above
A) Administration of an inappropriate drug
B) Misdiagnosis of an illness
C) Both of the above
D) Neither of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is pH a measure of?
A) The concentration of hydrogen ions in a solution
B) The normality of a solution containing 10 grams hydrogen ions in water
C) The reaction speed of hydrogen ions with bases in a solution
D) The specific gravity of 10 grams of hydrogen ions in water
A) The concentration of hydrogen ions in a solution
B) The normality of a solution containing 10 grams hydrogen ions in water
C) The reaction speed of hydrogen ions with bases in a solution
D) The specific gravity of 10 grams of hydrogen ions in water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
13
If a solution is held at a constant temperature of 25°C,at what pH would it be considered neutral?
A) 1
B) 6
C) 7
D) 10
A) 1
B) 6
C) 7
D) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
14
The concentration of a sodium chloride (NaCl)solution is defined as 10% NaCl (w/w).What does this tell us about the amount of NaCl in the solution?
A) There are 10 grams of NaCl in every 10% of solution.
B) There are 10 grams of NaCl in every 100 grams of solution.
C) There are 10 grams of NaCl in every 100 mL of solution.
D) There are 10 grams of NaCl in every 100 ounces of solution.
A) There are 10 grams of NaCl in every 10% of solution.
B) There are 10 grams of NaCl in every 100 grams of solution.
C) There are 10 grams of NaCl in every 100 mL of solution.
D) There are 10 grams of NaCl in every 100 ounces of solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
15
A vial of 3 mg silver chloride (AgCl)is poured into a flask containing 100 mL water and mixed.After 24 hours,2.9 mg of silver chloride remains as a solid at the bottom of the flask.Which of the following terms best describes the ability of silver chloride to dissolve in water?
A) Insoluble
B) Partly soluble
C) Soluble
D) Sparingly soluble
A) Insoluble
B) Partly soluble
C) Soluble
D) Sparingly soluble

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
16
Consider the following equation: concentration of glucose = 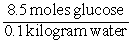
What are the units of the value calculated by this equation?
A) Molal
B) Molar
C) v/v
D) w/v
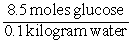
What are the units of the value calculated by this equation?
A) Molal
B) Molar
C) v/v
D) w/v

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
17
The concentration of an aqueous glucose solution is noted as 25% glucose (w/v).What does this tell us about the amount of glucose in the solution?
A) There are 0.25 grams of glucose in every 100 mL of solution.
B) There are 2.5 grams of glucose in every 100 mL of solution.
C) There are 25 grams of glucose in every 100 mL of solution.
D) There are 250 grams of glucose in every 100 mL of solution.
A) There are 0.25 grams of glucose in every 100 mL of solution.
B) There are 2.5 grams of glucose in every 100 mL of solution.
C) There are 25 grams of glucose in every 100 mL of solution.
D) There are 250 grams of glucose in every 100 mL of solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the number of equivalents of the base PO₄³⁻?
A) 2
B) 3
C) 4
D) 8
A) 2
B) 3
C) 4
D) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
19
A solution of aqueous ferric chloride has a concentration of 15 ppm.What does this tell us about the amount of ferric chloride in the solution?
A) There are 15 grams of ferric chloride in every 1,000,000 grams of solution.
B) There are 15 grams of ferric chloride in every 1,000,000 mL of solution.
C) There are 15 mL of ferric chloride in every 1,000,000 mL of solution.
D) Both A and B are correct.
A) There are 15 grams of ferric chloride in every 1,000,000 grams of solution.
B) There are 15 grams of ferric chloride in every 1,000,000 mL of solution.
C) There are 15 mL of ferric chloride in every 1,000,000 mL of solution.
D) Both A and B are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck








