Deck 1: Basic Physics for the Respiratory Therapist
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/107
العب
ملء الشاشة (f)
Deck 1: Basic Physics for the Respiratory Therapist
1
Incompressible substances that are able to maintain their volume and shape are called:
A) gases.
B) solids.
C) liquids.
D) compounds.
A) gases.
B) solids.
C) liquids.
D) compounds.
B
Solids are characterized as incompressible substances that can maintain their volume and shape.Gases and liquids do not maintain their volume and shape as well as solids do.
Solids are characterized as incompressible substances that can maintain their volume and shape.Gases and liquids do not maintain their volume and shape as well as solids do.
2
If the velocity of an object is reduced by half,its kinetic energy will be which of the following?
A) Reduced to one eighth
B) Increased twofold
C) Reduced twofold
D) Not changed
A) Reduced to one eighth
B) Increased twofold
C) Reduced twofold
D) Not changed
A
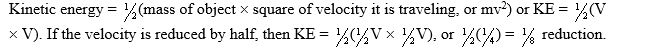
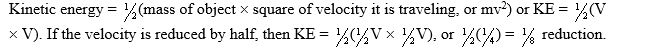
3
The most potential energy is contained by which state of matter?
A) Gases
B) Solids
C) Liquids
D) Mixtures
A) Gases
B) Solids
C) Liquids
D) Mixtures
B
Of all states of matter,solids contain the most potential energy; solids are followed by liquids and then gases.
Of all states of matter,solids contain the most potential energy; solids are followed by liquids and then gases.
4
The kinetic theory holds that:
A) all matter is composed of tiny particles.
B) elements combine in fixed proportions to form molecules.
C) the energy that an object gains as it falls is a result of gravity.
D) atoms and molecules that make up matter are in constant motion.
A) all matter is composed of tiny particles.
B) elements combine in fixed proportions to form molecules.
C) the energy that an object gains as it falls is a result of gravity.
D) atoms and molecules that make up matter are in constant motion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
5
Supercooled liquids are also known as which of the following?
A) Elements
B) Compounds
C) Crystalline solids
D) Amorphous solids
A) Elements
B) Compounds
C) Crystalline solids
D) Amorphous solids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
6
The potential energy of a compressed spring is known as which of the following?
A) Gravitational
B) Chemical
C) Inelastic
D) Elastic
A) Gravitational
B) Chemical
C) Inelastic
D) Elastic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which is the correct order for increasing size?
A) Atoms, molecules, mixtures, compounds, elements
B) Atoms, elements, molecules, compounds, mixtures
C) Elements, atoms, molecules, compounds, mixtures
D) Atoms, elements, mixtures, molecules, compounds
A) Atoms, molecules, mixtures, compounds, elements
B) Atoms, elements, molecules, compounds, mixtures
C) Elements, atoms, molecules, compounds, mixtures
D) Atoms, elements, mixtures, molecules, compounds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
8
Four horsepower (hp)is equal to how many kilowatts (kW)?
A) 5.36
B) 2.98
C) 2984
D) 186.5
A) 5.36
B) 2.98
C) 2984
D) 186.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
9
The energy that an object possesses when it is in motion is called:
A) sound.
B) kinetic.
C) thermal.
D) potential.
A) sound.
B) kinetic.
C) thermal.
D) potential.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following are amorphous solids?
1)Iron
2)Glass
3)Plastic
4)Margarine
A) 1
B) 1 and 3
C) 2 and 4
D) 2, 3, and 4
1)Iron
2)Glass
3)Plastic
4)Margarine
A) 1
B) 1 and 3
C) 2 and 4
D) 2, 3, and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
11
The least amount of kinetic energy is possessed by which one of the following?
A) Air
B) Iron
C) Water
D) Plastic
A) Air
B) Iron
C) Water
D) Plastic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
12
The weakest cohesive forces between constituent particles are present in which of the following?
A) Water
B) Plastic
C) Hydrogen
D) Liquid oxygen
A) Water
B) Plastic
C) Hydrogen
D) Liquid oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
13
Energy that is stored in an object is called which of the following?
A) Kinetic
B) Potential
C) Chemical
D) Mechanical
A) Kinetic
B) Potential
C) Chemical
D) Mechanical

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
14
When effort produces a change in the position of matter:
A) work is performed.
B) it is known as a joule.
C) mechanical power is created.
D) the law of the conservation of energy is being used.
A) work is performed.
B) it is known as a joule.
C) mechanical power is created.
D) the law of the conservation of energy is being used.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
15
The energy stored in heating oil is known as which of the following?
A) Elastic
B) Atomic
C) Chemical
D) Gravitational
A) Elastic
B) Atomic
C) Chemical
D) Gravitational

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
16
Power is expressed in which of the following units?
A) Newtons
B) Joules
C) Ohms
D) Watts
A) Newtons
B) Joules
C) Ohms
D) Watts

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
17
Power is a measure of which of the following?
A) Mechanical energy
B) Gravitational potential energy
C) The rate at which work is being performed
D) The rate at which atoms and molecules move
A) Mechanical energy
B) Gravitational potential energy
C) The rate at which work is being performed
D) The rate at which atoms and molecules move

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
18
The unit used to express the force of 1 newton (N)acting on a 1-kilogram (kg)object to move it 1 meter (m)is which of the following?
A) Watt
B) Joule
C) Kilowatt
D) Kinetic energy
A) Watt
B) Joule
C) Kilowatt
D) Kinetic energy

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
19
A cylinder of compressed gas contains 1500 psig at 70° F; the cylinder is heated to 120° F.Which of the following effects will occur as a result of the temperature change?
1)There will be increased kinetic activity in the contents of the cylinder
2)The volume of gas in the cylinder will increase
3)The pressure indicated on the pressure gauge will increase.
A) 1 only
B) 1 and 2
C) 1 and 3
D) 2 and 3
1)There will be increased kinetic activity in the contents of the cylinder
2)The volume of gas in the cylinder will increase
3)The pressure indicated on the pressure gauge will increase.
A) 1 only
B) 1 and 2
C) 1 and 3
D) 2 and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
20
Atoms and molecules arranged in an orderly fashion are called:
A) Solids
B) Mixtures
C) Crystalline
D) Amorphous
A) Solids
B) Mixtures
C) Crystalline
D) Amorphous

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
21
The temperature above which gas molecules cannot be converted back to a liquid,no matter how much pressure is exerted,is known as which of the following?
A) Critical temperature
B) Critical point
C) Boiling point
D) Latent heat
A) Critical temperature
B) Critical point
C) Boiling point
D) Latent heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
22
How many degrees Fahrenheit is 200° K?
A) -99.4° F
B) -58.3° F
C) 32° F
D) 0° F
A) -99.4° F
B) -58.3° F
C) 32° F
D) 0° F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
23
The point at which the vapor pressure of a liquid equals atmospheric pressure is known as which of the following?
A) Critical temperature
B) Vapor pressure
C) Boiling point
D) Latent heat
A) Critical temperature
B) Vapor pressure
C) Boiling point
D) Latent heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many degrees Kelvin is 25° F?
A) 298° K
B) 277° K
C) 269° K
D) 266° K
A) 298° K
B) 277° K
C) 269° K
D) 266° K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
25
Absolute zero is which of the following?
A) 0° K
B) The freezing point of water
C) Routinely measured in Fahrenheit
D) The temperature at which all molecular motion stops
A) 0° K
B) The freezing point of water
C) Routinely measured in Fahrenheit
D) The temperature at which all molecular motion stops

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
26
How much pressure must be applied to maintain equilibrium between liquid and gaseous oxygen at its critical temperature?
A) 1 atm
B) 37 atm
C) 43.9 atm
D) 49.7 atm
A) 1 atm
B) 37 atm
C) 43.9 atm
D) 49.7 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which two of the following are ways to enhance the process of evaporation?
1)Decrease the temperature of the liquid
2)Increase the temperature of the liquid
3)Decrease atmospheric pressure
4)Increase atmospheric pressure
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
1)Decrease the temperature of the liquid
2)Increase the temperature of the liquid
3)Decrease atmospheric pressure
4)Increase atmospheric pressure
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
28
A reduction in the force of gravity will cause the atmospheric pressure to:
A) shift.
B) increase.
C) decrease.
D) remain constant.
A) shift.
B) increase.
C) decrease.
D) remain constant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
29
How many degrees Fahrenheit is 425° K?
A) 152° F
B) 274° F
C) 306° F
D) 698° F
A) 152° F
B) 274° F
C) 306° F
D) 698° F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
30
How many degrees Celsius is 373° K?
A) 32° C
B) 100° C
C) 273° C
D) 341° C
A) 32° C
B) 100° C
C) 273° C
D) 341° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
31
The boiling point of liquid oxygen is which of the following?
A) -119° C
B) 182° F
C) -183° C
D) 49.7° C
A) -119° C
B) 182° F
C) -183° C
D) 49.7° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which two of the following temperatures are not equal?
1)15° C = 288° K
2)98.6° C = 32° F
3)20° F = -6.7° C
4)100° C = 273° K
A) 2 and 4
B) 1 and 3
C) 3 and 4
D) 1 and 2
1)15° C = 288° K
2)98.6° C = 32° F
3)20° F = -6.7° C
4)100° C = 273° K
A) 2 and 4
B) 1 and 3
C) 3 and 4
D) 1 and 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following statements are true concerning the latent heat of fusion?
1)It is also called evaporation.
2)It is expressed in calories per gram.
3)It will cause a complete change of state.
4)It is expressed in grams per degree Celsius.
A) 1 and 2
B) 1 and 3
C) 2 and 3
D) 2, 3, and 4
1)It is also called evaporation.
2)It is expressed in calories per gram.
3)It will cause a complete change of state.
4)It is expressed in grams per degree Celsius.
A) 1 and 2
B) 1 and 3
C) 2 and 3
D) 2, 3, and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
34
How many degrees Fahrenheit is 100° K?
A) -331° F
B) -279° F
C) -173° F
D) 212° F
A) -331° F
B) -279° F
C) -173° F
D) 212° F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many millimeters of mercury is 25 cm H₂O?
A) 2.45
B) 18.4
C) 188
D) 34
A) 2.45
B) 18.4
C) 188
D) 34

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
36
The process whereby a solid directly becomes a gas is known as:
A) latent heat.
B) sublimation.
C) evaporation.
D) condensation.
A) latent heat.
B) sublimation.
C) evaporation.
D) condensation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many kilopascals are equal to 15 mm Hg?
A) 2
B) 11
C) 153
D) 1.47
A) 2
B) 11
C) 153
D) 1.47

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which two of the following are considered vapors?
1)Carbon dioxide
2)Nitrogen
3)Oxygen
4)Water
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
1)Carbon dioxide
2)Nitrogen
3)Oxygen
4)Water
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
39
How many degrees Celsius is 101° F?
A) 24° C
B) 145° C
C) 38.3° C
D) 56.1° C
A) 24° C
B) 145° C
C) 38.3° C
D) 56.1° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
40
The temperature at which a solid converts to a liquid is the _____ point.
A) freezing
B) melting
C) boiling
D) critical
A) freezing
B) melting
C) boiling
D) critical

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
41
If temperature is constant,which pressure results in the largest volume?
A) 15 mm Hg
B) 760 mm Hg
C) 1520 mm Hg
D) 2000 mm Hg
A) 15 mm Hg
B) 760 mm Hg
C) 1520 mm Hg
D) 2000 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
42
According to Laplace's law,if the surface tension of a sphere is doubled,what will happen to the pressure within the sphere?
A) The pressure will decrease by one half.
B) The pressure will increase by one half.
C) The pressure will quadruple.
D) The pressure will double.
A) The pressure will decrease by one half.
B) The pressure will increase by one half.
C) The pressure will quadruple.
D) The pressure will double.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
43
Viscosity has an influence on which of the following?
1)Laminar flow
2)Fluid mechanics
3)Sedimentary rate
4)Streamlined flow
A) 1
B) 2 and 3
C) 2 and 4
D) 1,2,and 4
1)Laminar flow
2)Fluid mechanics
3)Sedimentary rate
4)Streamlined flow
A) 1
B) 2 and 3
C) 2 and 4
D) 1,2,and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
44
Boyle's law describes the relationship between which of the following?
A) Pressure and temperature
B) Volume and temperature
C) Volume and pressure
D) Pressure and density
A) Pressure and temperature
B) Volume and temperature
C) Volume and pressure
D) Pressure and density

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
45
Forces at the molecular interface between oil and water are known as _____ forces.
A) van der Waals
B) Hydrostatic
C) Cohesive
D) Adhesive
A) van der Waals
B) Hydrostatic
C) Cohesive
D) Adhesive

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
46
What will happen to the surface tension of water droplets when a surface-active agent is added?
A) Nothing will happen.
B) It will increase.
C) It will decrease.
D) It will be eliminated.
A) Nothing will happen.
B) It will increase.
C) It will decrease.
D) It will be eliminated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which substance has the lowest surface tension?
A) Water at 20° C
B) Water at 37° C
C) Blood at 37° C
D) Ethyl alcohol at 20° C
A) Water at 20° C
B) Water at 37° C
C) Blood at 37° C
D) Ethyl alcohol at 20° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
48
Under what conditions is the relationship between mass and weight constant?
A) In outer space
B) At zero gravity
C) At the center of the Earth
D) Near the surface of the Earth
A) In outer space
B) At zero gravity
C) At the center of the Earth
D) Near the surface of the Earth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
49
Specific gravity is best described as which of the following?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
50
According to the Système International d'Unités,surface tension is measured in:
A) cc3.
B) lb/iN₂.
C) lb/cc3.
D) dyne/cm.
A) cc3.
B) lb/iN₂.
C) lb/cc3.
D) dyne/cm.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following shows the correct relationship among density,volume,and mass?
1)Density = volume/mass
2)Volume = density/mass
3)Mass = (density)/(volume)
4)Weight density = weight/volume
A) 1 and 3
B) 1 and 4
C) 4
D) 3 and 4
1)Density = volume/mass
2)Volume = density/mass
3)Mass = (density)/(volume)
4)Weight density = weight/volume
A) 1 and 3
B) 1 and 4
C) 4
D) 3 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
52
A small-diameter glass tube is placed upright in a container of mercury.The meniscus at the top of the column of mercury is convex.This demonstrates that the:
A) cohesive forces of mercury are weak.
B) cohesive forces of mercury are strong.
C) adhesive forces within the mercury are strong.
D) adhesive forces between the mercury and the glass are strong.
A) cohesive forces of mercury are weak.
B) cohesive forces of mercury are strong.
C) adhesive forces within the mercury are strong.
D) adhesive forces between the mercury and the glass are strong.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
53
Surface tension is present in a container with which of the following?
A) Oxygen and hydrogen
B) Water and mercury
C) Water and chlorine
D) Water and salt
A) Oxygen and hydrogen
B) Water and mercury
C) Water and chlorine
D) Water and salt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which of the following is least viscous?
A) Plastic
B) Gelatin (e.g., Jell-O)
C) Metal
D) Glass
A) Plastic
B) Gelatin (e.g., Jell-O)
C) Metal
D) Glass

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
55
For solids and liquids,density can be expressed in which of the following units?
1)g/L
2)mg/mL
3)g/cc
4)L/cc
A)1
1)g/L
2)mg/mL
3)g/cc
4)L/cc
A)1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
56
A hydrometer is usually associated with measuring which of the following?
1)Hydrogen content
2)Specific gravity
3)Weight density
4)Water vapor
A) 1 and 3
B) 2 and 4
C) 2 and 3
D) 1,2,and 3
1)Hydrogen content
2)Specific gravity
3)Weight density
4)Water vapor
A) 1 and 3
B) 2 and 4
C) 2 and 3
D) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
57
Properties of a viscous liquid are:
1)Increased cohesive forces between its molecules
2)Low density
3)High density
4)Free flow
A) 1 and 3
B) 1,2,and 4
C) 2 and 4
D) 3 and 4
1)Increased cohesive forces between its molecules
2)Low density
3)High density
4)Free flow
A) 1 and 3
B) 1,2,and 4
C) 2 and 4
D) 3 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
58
What are the two opposing forces in a mercury barometer?
A) The weight of the mercury column and the force of the gas molecules
B) The weight of the mercury column and the spring tension
C) The spring tension and the gas pressure
D) The gravity and the gas pressure
A) The weight of the mercury column and the force of the gas molecules
B) The weight of the mercury column and the spring tension
C) The spring tension and the gas pressure
D) The gravity and the gas pressure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
59
The effects of buoyancy are best explained by:
A) Archimedes principle.
B) Bernoulli principle.
C) Dalton's law.
D) Boyle's law.
A) Archimedes principle.
B) Bernoulli principle.
C) Dalton's law.
D) Boyle's law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
60
The surface tension of a liquid:
A) does not vary with temperature.
B) increases as temperature increases.
C) increases as temperature decreases.
D) decreases as temperature increases.
A) does not vary with temperature.
B) increases as temperature increases.
C) increases as temperature decreases.
D) decreases as temperature increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the following does not follow Dalton's law at sea level?
A) Oxygen
B) Nitrogen
C) Water vapor
D) Trace gases
A) Oxygen
B) Nitrogen
C) Water vapor
D) Trace gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which gas law describes the relationship between the temperature and pressure of a gas when volume is constant?
A) Gay-Lussac's law
B) Charles' law
C) Dalton's law
D) Boyle's law
A) Gay-Lussac's law
B) Charles' law
C) Dalton's law
D) Boyle's law

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
63
The direct relationship between the volume and temperature of a gas is the basic principle of _____ law.
A) Gay-Lussac's
B) Charles'
C) Dalton's
D) Boyle's
A) Gay-Lussac's
B) Charles'
C) Dalton's
D) Boyle's

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
64
The partial pressure of oxygen when there is 25% oxygen in a gas mixture at an atmospheric pressure of 760 mm Hg is ____ mm Hg.
A) 190
B) 30.4
C) 1900
D) 159.6
A) 190
B) 30.4
C) 1900
D) 159.6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
65
It is implied that the absolute temperature of a gas will rise as the pressure is increased when which of the following occurs?
A) Absolute temperature of the gas reaches absolute zero
B) Size of the container remains constant
C) Volume of the gas is held constant
D) Volume of the gas is increased
A) Absolute temperature of the gas reaches absolute zero
B) Size of the container remains constant
C) Volume of the gas is held constant
D) Volume of the gas is increased

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which gas has the lowest specific gravity at 25° C and 760 mm Hg?
A) Water vapor
B) Helium
C) CO₂
D) O₂
A) Water vapor
B) Helium
C) CO₂
D) O₂

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
67
The movement of gas molecules from an area of high concentration to one of lower concentration describes the property of which of the following?
A) Osmosis
B) Effusion
C) Diffusion
D) Suspension
A) Osmosis
B) Effusion
C) Diffusion
D) Suspension

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
68
In the combined-gas law,n represents:
A) Boltzmann's universal gas constant.
B) the atomic mass of the gas.
C) the number of moles of gas.
D) the partial pressure of a gas.
A) Boltzmann's universal gas constant.
B) the atomic mass of the gas.
C) the number of moles of gas.
D) the partial pressure of a gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
69
One mole of any gas will occupy 22.4 L and contain ________ molecules.
A) 6.02 * 1023
B) 6.2 * 1023
C) 0.602 * 1023
D) 6.2 * 10-23
A) 6.02 * 1023
B) 6.2 * 1023
C) 0.602 * 1023
D) 6.2 * 10-23

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following formulas represents Boyle's law?
A) V = 2P
B) V = 1/2P
C) P1V1 = P2V2
D) P1/P2 = V1/V2
A) V = 2P
B) V = 1/2P
C) P1V1 = P2V2
D) P1/P2 = V1/V2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
71
At what temperature would you expect to see the highest water-vapor pressure?
A) 0° C
B) 40° C
C) 100° C
D) Absolute zero
A) 0° C
B) 40° C
C) 100° C
D) Absolute zero

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which is the correct formula for the principles of the combined-gas law?
A) P1V1/T1 = P2V2/T2
B) PVT = nR
C) P1V1/T2 = P2V2/T1
D) T2/P1V1 = T1/P2V2
A) P1V1/T1 = P2V2/T2
B) PVT = nR
C) P1V1/T2 = P2V2/T1
D) T2/P1V1 = T1/P2V2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
73
The combined-gas law best describes which of the following?
A) The behavior of all gases when volume is constant
B) The combined behavior of pressure, volume, and temperature
C) The additive properties of individual gases occupying the same space
D) The macroscopic behavior of gases when any or all variables change simultaneously
A) The behavior of all gases when volume is constant
B) The combined behavior of pressure, volume, and temperature
C) The additive properties of individual gases occupying the same space
D) The macroscopic behavior of gases when any or all variables change simultaneously

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
74
A practical application of Avogadro's law is seen in the calculation of which of the following?
1)Specific gravity
2)Diffusion rate
3)Gas density
4)Osmosis
A) 1 and 2
B) 1 and 3
C) 2 and 4
D) 3 and 4
1)Specific gravity
2)Diffusion rate
3)Gas density
4)Osmosis
A) 1 and 2
B) 1 and 3
C) 2 and 4
D) 3 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
75
The sum of the partial pressures of a gas mixture equals the total gas pressure of the system.This statement represents which of the following laws?
A) Dalton's law
B) Avogadro's law
C) The combined-gas law
D) Boltzmann's Universal Gas Constant
A) Dalton's law
B) Avogadro's law
C) The combined-gas law
D) Boltzmann's Universal Gas Constant

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following formulas represents Gay-Lussac's law?
A) P1/T1 = T2/P2
B) P1T1 = P2T2
C) P1T2 = P2T1
D) P = 1/T
A) P1/T1 = T2/P2
B) P1T1 = P2T2
C) P1T2 = P2T1
D) P = 1/T

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
77
The partial pressure of a gas can be obtained by doing which of the following?
A) Multiplying the total mixture pressure by the percentage area a particular gas occupies
B) Multiplying the atmospheric pressure by the percentage of water vapor present
C) Subtracting the partial pressure of water vapor from the atmospheric pressure
D) Dividing the total pressure of a gas mixture by the atmospheric pressure
A) Multiplying the total mixture pressure by the percentage area a particular gas occupies
B) Multiplying the atmospheric pressure by the percentage of water vapor present
C) Subtracting the partial pressure of water vapor from the atmospheric pressure
D) Dividing the total pressure of a gas mixture by the atmospheric pressure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
78
The relationship of how the volume of a gas varies with temperature is known as _____ law.
A) Gay-Lussac's
B) Newton's
C) Charles'
D) Boyle's
A) Gay-Lussac's
B) Newton's
C) Charles'
D) Boyle's

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
79
The partial pressure of nitrogen at 1 atm is _____ mm Hg.
A) 661.2
B) 592.8
C) 159.6
D) 0.228
A) 661.2
B) 592.8
C) 159.6
D) 0.228

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
80
The molecular weight of a gas divided by 22.4 L is used to express which of the following?
A) Density
B) Diffusion rate
C) Partial pressure
D) Specific gravity
A) Density
B) Diffusion rate
C) Partial pressure
D) Specific gravity

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck








