Deck 1: Acids, Bases, Structure and Bonding
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/13
العب
ملء الشاشة (f)
Deck 1: Acids, Bases, Structure and Bonding
1
Which of the following statements about resonance structures is true?
A) Resonance structures have the same placement of atoms but different arrangement of electrons.
B) Resonance structures have the same placement of electrons but different arrangement of atoms.
C) Resonance structures have different placement of atoms and different arrangement of electrons.
D) Resonance structures have the same placement of atoms and the same arrangement of electrons.
A) Resonance structures have the same placement of atoms but different arrangement of electrons.
B) Resonance structures have the same placement of electrons but different arrangement of atoms.
C) Resonance structures have different placement of atoms and different arrangement of electrons.
D) Resonance structures have the same placement of atoms and the same arrangement of electrons.
Resonance structures have the same placement of atoms but different arrangement of electrons.
2
Which is the correct Lewis structure for acetic acid (CH3CO2H)? 
A) III
B) I
C) IV
D) II

A) III
B) I
C) IV
D) II
IV
3
Which of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules?
A) Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds, and oxygen forms 2 covalent bonds.
B) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds, and oxygen forms 4 covalent bonds.
C) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds, and oxygen forms 2 covalent bonds.
D) Carbon forms 4 covalent bonds, nitrogen forms 2 covalent bonds, and oxygen forms 3 covalent bonds.
A) Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds, and oxygen forms 2 covalent bonds.
B) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds, and oxygen forms 4 covalent bonds.
C) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds, and oxygen forms 2 covalent bonds.
D) Carbon forms 4 covalent bonds, nitrogen forms 2 covalent bonds, and oxygen forms 3 covalent bonds.
Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds, and oxygen forms 2 covalent bonds.
4
Which of the following molecules does not have a net dipole moment of zero?
A) BF3
B) CO2
C) NH3
D) CCl4
A) BF3
B) CO2
C) NH3
D) CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which atomic orbitals overlap to form the carbon-carbon s and p bonding molecular orbitals of ethylene, H2C=CH2?
A) Csp3 + Csp3, and C2p + C2p
B) Csp3 + Csp3, and Csp2 + Csp2
C) Csp2 + Csp2, and C2p + C2p
D) Csp2 + Csp2, and Csp2 + Csp2
A) Csp3 + Csp3, and C2p + C2p
B) Csp3 + Csp3, and Csp2 + Csp2
C) Csp2 + Csp2, and C2p + C2p
D) Csp2 + Csp2, and Csp2 + Csp2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following pair does not represent resonance structures? 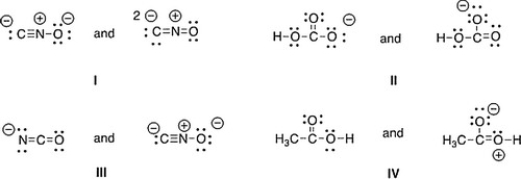
A) IV
B) II
C) III
D) I

A) IV
B) II
C) III
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which is not an acceptable Lewis structure for the anion CH2NCO-? 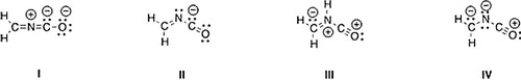
A) II
B) III
C) I
D) IV
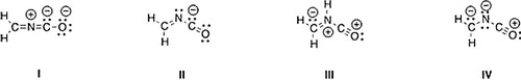
A) II
B) III
C) I
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the ground-state electronic configuration of a chlorine anion (Cl-)?
A) 1s2, 2s2, 2p6
B) 1s2, 2s2, 2p6, 3s2, 3p5
C) 1s2, 2s2, 2p6, 3s2, 3p4
D) 1s2, 2s2, 2p6, 3s2, 3p6
A) 1s2, 2s2, 2p6
B) 1s2, 2s2, 2p6, 3s2, 3p5
C) 1s2, 2s2, 2p6, 3s2, 3p4
D) 1s2, 2s2, 2p6, 3s2, 3p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following compounds has an atom with more than eight valence electrons?
A) H2CO3
B) H2SO4
C) HBr
D) H2O
A) H2CO3
B) H2SO4
C) HBr
D) H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
10
Avobenzone is an active ingredient in some common sunscreens. Which of the following is the correct molecular formula for avobenzone? 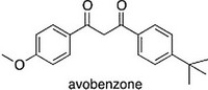
A) C20H24O3
B) C20H22O3
C) C21H23O3
D) C22O24O3

A) C20H24O3
B) C20H22O3
C) C21H23O3
D) C22O24O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the hybridization for each of the indicated atoms in the following compound? 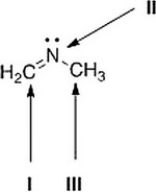
A) I = sp; II = sp2; III = sp3.
B) I = sp2; II = sp2; III = sp3.
C) I = sp2; II = sp3; III = sp3.
D) I = sp2; II = sp2; III = sp2.

A) I = sp; II = sp2; III = sp3.
B) I = sp2; II = sp2; III = sp3.
C) I = sp2; II = sp3; III = sp3.
D) I = sp2; II = sp2; III = sp2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
12
Determine the geometry around the indicated atom in each species. 
A) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
B) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
C) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
D) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear

A) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
B) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
C) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
D) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following statements about resonance structures is not true?
A) Resonance structures differ only in the arrangement of electrons.
B) There is no movement of electrons from one form to another.
C) Resonance structures are not isomers.
D) Resonance structures are in equilibrium with each other.
A) Resonance structures differ only in the arrangement of electrons.
B) There is no movement of electrons from one form to another.
C) Resonance structures are not isomers.
D) Resonance structures are in equilibrium with each other.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 13 في هذه المجموعة.
فتح الحزمة
k this deck








