Deck 18: Benzene and Aromatic Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/10
العب
ملء الشاشة (f)
Deck 18: Benzene and Aromatic Compounds
1
Which of the following compounds is not aromatic? 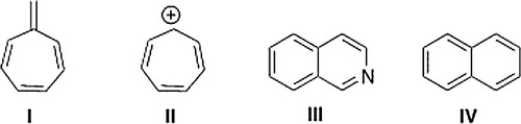
A) I
B) IV
C) II
D) III

A) I
B) IV
C) II
D) III
I
2
Which of the following compounds is aromatic? 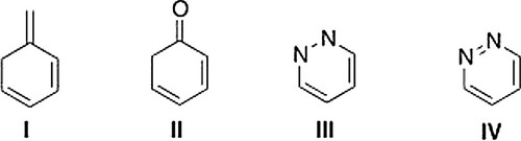
A) I
B) IV
C) II
D) III

A) I
B) IV
C) II
D) III
IV
3
Why is the following compound not aromatic? 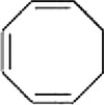
A) It is not cyclic.
B) The pi electron system is not continuous.
C) It has 4n+2 pi electrons.
D) It has 4n pi electrons.

A) It is not cyclic.
B) The pi electron system is not continuous.
C) It has 4n+2 pi electrons.
D) It has 4n pi electrons.
The pi electron system is not continuous.
4
What is the name of the following compound? 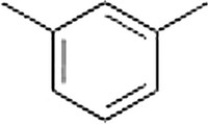
A) 5-Methyltoluene
B) 2,6-Dimethylbenzene
C) Anisole
D) 1,3-Dimethylbenzene

A) 5-Methyltoluene
B) 2,6-Dimethylbenzene
C) Anisole
D) 1,3-Dimethylbenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following compounds is aromatic? 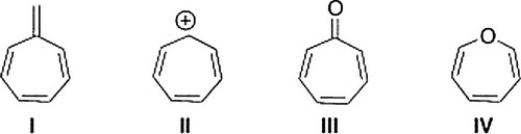
A) II
B) IV
C) III
D) I

A) II
B) IV
C) III
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following statements about the molecular orbital (MO) theory is true? 
A) Ap* antibonding molecular orbital is higher in energy than the two atomicp orbitals from which it is formed.
B) When twop orbitals of similar phase overlap side-by-side, a p* antibonding molecular orbital is formed.
C) Ap bonding molecular orbital is higher in energy than the two atomicp orbitals from which it is formed.
D) When twop orbitals of opposite phase overlap side-by-side, a p bonding molecular orbital is formed.

A) Ap* antibonding molecular orbital is higher in energy than the two atomicp orbitals from which it is formed.
B) When twop orbitals of similar phase overlap side-by-side, a p* antibonding molecular orbital is formed.
C) Ap bonding molecular orbital is higher in energy than the two atomicp orbitals from which it is formed.
D) When twop orbitals of opposite phase overlap side-by-side, a p bonding molecular orbital is formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the correct assignment of the number of signals in the 13C NMR spectra of the following disubstituted benzene derivatives? 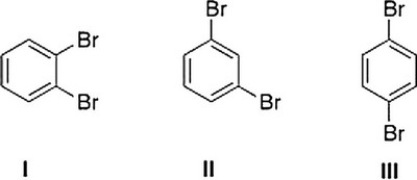
A) I = 6 signals; II = 6 signals; III = 6 signals
B) I = 3 signals; II = 4 signals; III = 2 signals
C) I = 3 signals; II = 4 signals; III = 3 signals
D) I = 3 signals; II = 3 signals; III = 2 signals

A) I = 6 signals; II = 6 signals; III = 6 signals
B) I = 3 signals; II = 4 signals; III = 2 signals
C) I = 3 signals; II = 4 signals; III = 3 signals
D) I = 3 signals; II = 3 signals; III = 2 signals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following compounds is not aromatic? 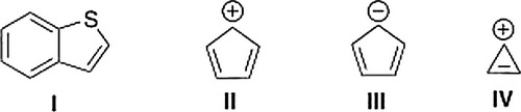
A) III
B) II
C) IV
D) I

A) III
B) II
C) IV
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the correct assignment of the names of the following fused aromatic compounds? ![<strong>What is the correct assignment of the names of the following fused aromatic compounds? </strong> A) I = naphthalene; II = phenanthrene; III = anthracene B) I = naphthalene; II = anthracene; III = phenanthrene C) I = anthracene; II = naphthalene; III = phenanthrene D) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfe_614c_862d_8b402207188d_TBMG1035_00.jpg)
A) I = naphthalene; II = phenanthrene; III = anthracene
B) I = naphthalene; II = anthracene; III = phenanthrene
C) I = anthracene; II = naphthalene; III = phenanthrene
D) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene
![<strong>What is the correct assignment of the names of the following fused aromatic compounds? </strong> A) I = naphthalene; II = phenanthrene; III = anthracene B) I = naphthalene; II = anthracene; III = phenanthrene C) I = anthracene; II = naphthalene; III = phenanthrene D) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene](https://d2lvgg3v3hfg70.cloudfront.net/TBMG1035/11ee4665_5cfe_614c_862d_8b402207188d_TBMG1035_00.jpg)
A) I = naphthalene; II = phenanthrene; III = anthracene
B) I = naphthalene; II = anthracene; III = phenanthrene
C) I = anthracene; II = naphthalene; III = phenanthrene
D) I = naphthalene; II = phenanthrene; III = benzo[a]pyrene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the IUPAC name of the following compound? 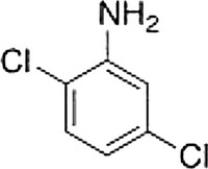
A) 2,5-Dichloroaminobenzene
B) 2,5-Dichloroaniline
C) 2,5-Dichloroanisole
D) 3,6-Dichloroaniline

A) 2,5-Dichloroaminobenzene
B) 2,5-Dichloroaniline
C) 2,5-Dichloroanisole
D) 3,6-Dichloroaniline

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck








