Deck 5: Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/90
العب
ملء الشاشة (f)
Deck 5: Reactions
1
What will you observe if you mix a solution of KOH with a solution of MgI2 in the reaction below? 
A) bubbles of KI will form
B) a solid of Mg(OH)2 will settle in reaction vessel
C) a solid of KOH will form
D) a clear solution will form

A) bubbles of KI will form
B) a solid of Mg(OH)2 will settle in reaction vessel
C) a solid of KOH will form
D) a clear solution will form
a solid of Mg(OH)2 will settle in reaction vessel
2
Which set of coefficients properly balance the equation below, going from reactants to products? 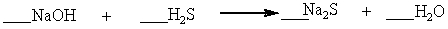
A) 1,2,1,4
B) 2,1,1,2
C) 2,2,3,1
D) 1,2,2,1
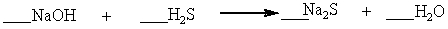
A) 1,2,1,4
B) 2,1,1,2
C) 2,2,3,1
D) 1,2,2,1
2,1,1,2
3
Lithium is a metal that reacts vigorously with oxygen. Which set of coefficents balance the equation below? 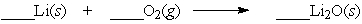
A) 1,2,1
B) 2,2,1
C) 1,2,2
D) 4,1,2
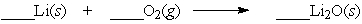
A) 1,2,1
B) 2,2,1
C) 1,2,2
D) 4,1,2
4,1,2
4
In chemical reaction
A) the covalent bonds and ionic bonds that hold elements and compounds together remain intact as new bonds are formed
B) the covalent and ionic bonds that hold elements and compounds together are broken and no new bonds are formed
C) the covalent and ionic bonds that hold elements and compounds together are broken and new bonds are formed
D) neither new bonds are formed nor old bonds broken
A) the covalent bonds and ionic bonds that hold elements and compounds together remain intact as new bonds are formed
B) the covalent and ionic bonds that hold elements and compounds together are broken and no new bonds are formed
C) the covalent and ionic bonds that hold elements and compounds together are broken and new bonds are formed
D) neither new bonds are formed nor old bonds broken

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
5
Balance the equation and list the coefficients in the balanced equation below, going from reactants to products. 
A) 2,1,1,1
B) 2,2,1,1
C) 1,1,2,1
D) 1,2,1,2

A) 2,1,1,1
B) 2,2,1,1
C) 1,1,2,1
D) 1,2,1,2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
6
When properly balanced with whole number coefficients, what is the coefficient on H2O?
____HCl + ____Ba(OH)2 ___BaCl2 + ___H2O
A) 1
B) 2
C) 3
D) 6
____HCl + ____Ba(OH)2 ___BaCl2 + ___H2O
A) 1
B) 2
C) 3
D) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
7
When properly balanced with whole number coefficients, what is the coefficient on N2 ? 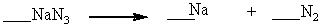
A) 4
B) 1
C) 2
D) 3
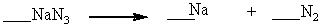
A) 4
B) 1
C) 2
D) 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
8
Aluminum will react slowly with the oxygen in the air. When the equation below is balanced, with whole number coefficients, what is the coefficient on aluminum oxide? 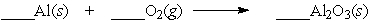
A) 4
B) 3
C) 2
D) 1
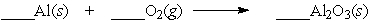
A) 4
B) 3
C) 2
D) 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
9
Pentose, C5H10O5, reacts with oxygen to produce carbon dioxide and water. What is the coefficient on O2 when the equation below is properly balanced? 
A) 3
B) 5
C) 7
D) 15

A) 3
B) 5
C) 7
D) 15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which set of coefficients properly balance the equation below? 
A) 1,1,5,6
B) 1,5,5,6
C) 1,5,5,1
D) 1,8,5,6

A) 1,1,5,6
B) 1,5,5,6
C) 1,5,5,1
D) 1,8,5,6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which set of coefficients properly balance the equation below, going from reactants to products? 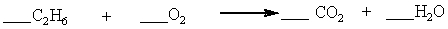
A) 1,3,2,3
B) 1,7,4,3
C) 2,7,4,6
D) 2,3,2,3
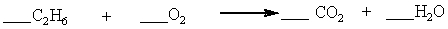
A) 1,3,2,3
B) 1,7,4,3
C) 2,7,4,6
D) 2,3,2,3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
12
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 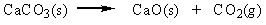
A) single replacement
B) double replacement
C) decomposition
D) synthesis
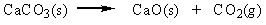
A) single replacement
B) double replacement
C) decomposition
D) synthesis

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
13
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 
A) single replacement
B) double replacement
C) synthesis
D) decomposition

A) single replacement
B) double replacement
C) synthesis
D) decomposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
14
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 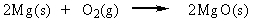
A) double replacement
B) single replacement
C) decomposition
D) synthesis
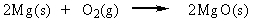
A) double replacement
B) single replacement
C) decomposition
D) synthesis

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
15
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 
A) synthesis
B) single replacement
C) double replacement
D) decomposition

A) synthesis
B) single replacement
C) double replacement
D) decomposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
16
The following equation is an example of a ___ reaction. 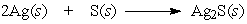
A) synthesis
B) decomposition
C) single replacement
D) double replacement
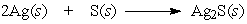
A) synthesis
B) decomposition
C) single replacement
D) double replacement

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
17
The reaction below is an example of a ___ reaction. 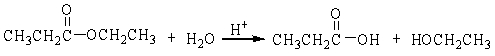
A) hydrogenation
B) hydrolysis
C) dehydration
D) neutralization
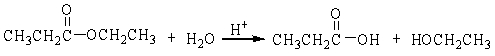
A) hydrogenation
B) hydrolysis
C) dehydration
D) neutralization

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
18
The reaction below is an example of a ___ reaction. 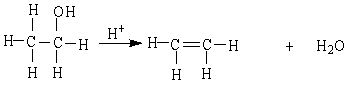
A) dehydration
B) hydrogenation
C) hydrolysis
D) combustion

A) dehydration
B) hydrogenation
C) hydrolysis
D) combustion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
19
The reaction below is an example of a ___ reaction. 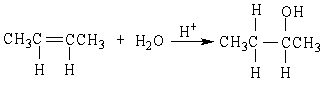
A) hydrogenation
B) hydrolysis
C) dehydration
D) hydration
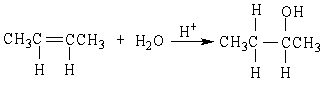
A) hydrogenation
B) hydrolysis
C) dehydration
D) hydration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
20
If ethene (H2C=CH2) were to react with water in an acid solution (H+ present), the result would be which of the following?
A)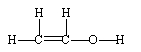
B)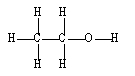
C)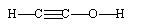
D)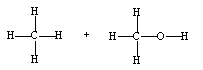
A)

B)

C)
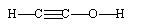
D)
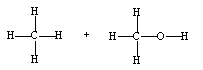

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
21
The missing reactant is 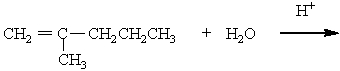
A)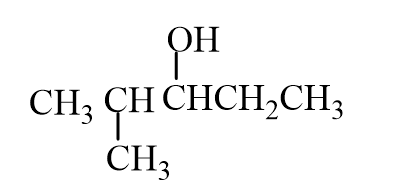
B)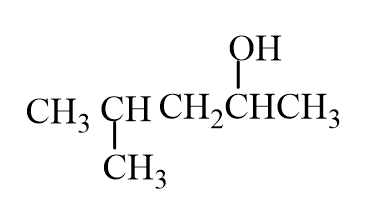
C)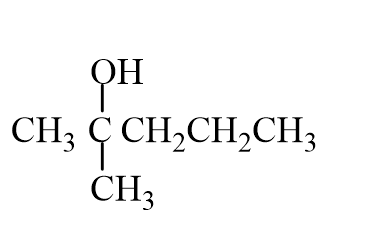
D)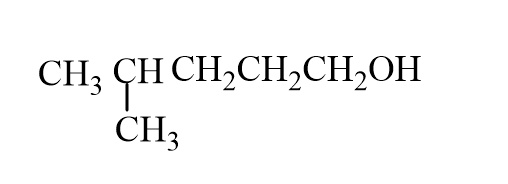
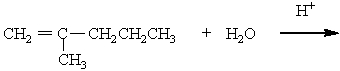
A)
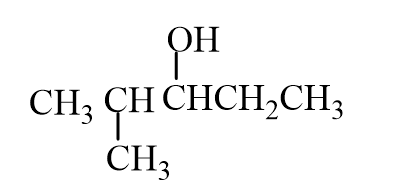
B)
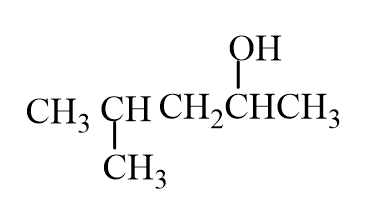
C)
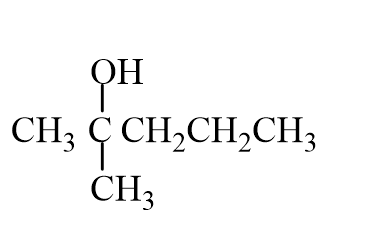
D)
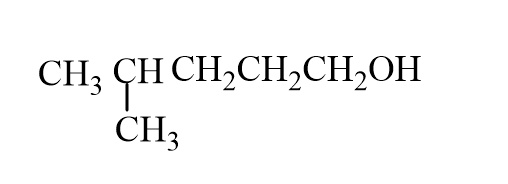

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
22
2-methylcyclopentene reacts with hydrogen in the presence of platinum catalyst to form methylcyclopentane.
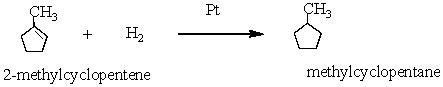 In this reaction:
In this reaction:
A) 2-methylcyclopentene is reduced
B) Pt is oxidized
C) methylcyclopentane is reduced
D) cyclohexane is oxidized
 In this reaction:
In this reaction:A) 2-methylcyclopentene is reduced
B) Pt is oxidized
C) methylcyclopentane is reduced
D) cyclohexane is oxidized

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
23
A chemical reaction in which there is a transfer of electrons from one reactant to another reactant can be classified as
A) decomposition reaction
B) combustion reaction
C) oxidation-reduction reaction
D) double replacement reaction
A) decomposition reaction
B) combustion reaction
C) oxidation-reduction reaction
D) double replacement reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
24
In the reaction below, which element is oxidized? 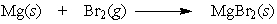
A) Mg
B) Br2
C) neither Mg nor Br2 is oxidized
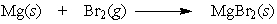
A) Mg
B) Br2
C) neither Mg nor Br2 is oxidized

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
25
In the reaction below, _____. 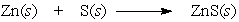
A) Zn and S are both oxidized
B) Zn and S are both reduced
C) Zn is reduced and S is oxidized
D) Zn is oxidized and S is reduced
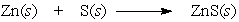
A) Zn and S are both oxidized
B) Zn and S are both reduced
C) Zn is reduced and S is oxidized
D) Zn is oxidized and S is reduced

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
26
In the reaction below: 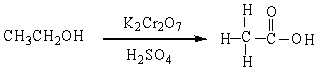
A) CH3CH2OH is oxidized
B) CH3CH2OH is reduced
C)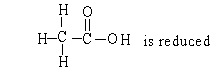
D) CH3CH2OH in neither oxidized nor reduced
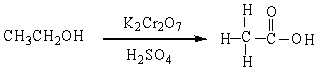
A) CH3CH2OH is oxidized
B) CH3CH2OH is reduced
C)
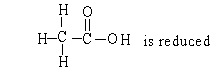
D) CH3CH2OH in neither oxidized nor reduced

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
27
In the reaction below: 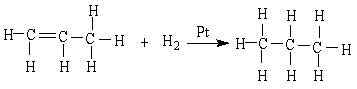
A) CH3CH=CH2 is oxidized
B) CH3CH=CH2 is reduced
C) CH3CH2CH3 is reduced
D) CH3CH=CH2 is neither oxidized nor reduced
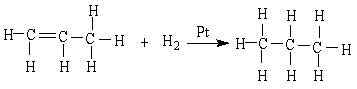
A) CH3CH=CH2 is oxidized
B) CH3CH=CH2 is reduced
C) CH3CH2CH3 is reduced
D) CH3CH=CH2 is neither oxidized nor reduced

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
28
Barium and sulfur react to produce barium sulfide. What is the role of barium in this reaction? 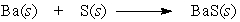
A) Barium is not affected by the processes of the chemical reaction.
B) Barium is being reduced.
C) Barium serves as the reducing agent.
D) Barium is the oxidizing agent.
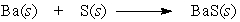
A) Barium is not affected by the processes of the chemical reaction.
B) Barium is being reduced.
C) Barium serves as the reducing agent.
D) Barium is the oxidizing agent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
29
Regarding the following reaction, 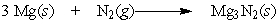
A) This reaction does not involve oxidation or reduction.
B) Magnesium is oxidized and nitrogen is reduced.
C) Both magnesium and nitrogen are reduced.
D) Magnesium is reduced and nitrogen is oxidized.
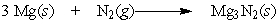
A) This reaction does not involve oxidation or reduction.
B) Magnesium is oxidized and nitrogen is reduced.
C) Both magnesium and nitrogen are reduced.
D) Magnesium is reduced and nitrogen is oxidized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
30
What was the earliest reported method of fighting infection in the operating room?
A) The patient was treated with Betadine before surgery.
B) Carbolic acid was used on the surgeon's tools.
C) Listerine was the antiseptic of choice.
D) The surgeons washed with soap and chlorine water.
A) The patient was treated with Betadine before surgery.
B) Carbolic acid was used on the surgeon's tools.
C) Listerine was the antiseptic of choice.
D) The surgeons washed with soap and chlorine water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
31
Benzoyl peroxide can be used as a treatment for acne. Benzoyl peroxide
A) has a reduction action that tends to neutralize the effects of the acne bacteria.
B) is injected so that it will have a general immune action against bacteria causing acne.
C) is an oxidizing agent used on the skin to kill the bacteria causing acne.
D) is not used for acne; it is an antiseptic used to sterilize a surgeon's hands.
A) has a reduction action that tends to neutralize the effects of the acne bacteria.
B) is injected so that it will have a general immune action against bacteria causing acne.
C) is an oxidizing agent used on the skin to kill the bacteria causing acne.
D) is not used for acne; it is an antiseptic used to sterilize a surgeon's hands.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which reaction is NOT an oxidation-reduction reaction?
A)
B)
C)
D) All of these are oxidation-reduction reactions.
A)

B)

C)

D) All of these are oxidation-reduction reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
33
Ethyne, C2H2, used in welding react with oxygen as shown below.
 Which of the following is about this reaction?
Which of the following is about this reaction?
A) carbon is oxidized
B) the reaction can be classified as a hydration reaction
C) C2H2 is the oxidizing agent
D) CO2 is the reducing agent
 Which of the following is about this reaction?
Which of the following is about this reaction?A) carbon is oxidized
B) the reaction can be classified as a hydration reaction
C) C2H2 is the oxidizing agent
D) CO2 is the reducing agent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
34
Isooctane, C8H18, a component of gasoline is a liquid at room temperature. How many moles of oxygen are required to completely react with 4 moles of isooctane? 
A) 50
B) 12
C) 100
D) 25

A) 50
B) 12
C) 100
D) 25

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
35
In the following reaction, the aldehyde has been 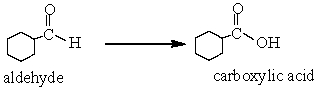
A) Reduced
B) Oxidized
C) Hydrolysed
D) None of these choices.

A) Reduced
B) Oxidized
C) Hydrolysed
D) None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
36
In the following reaction, 10.0 moles of potassium would require how many moles of bromine? 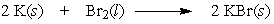
A) 2.00
B) 5.00
C) 10.0
D) 20.0
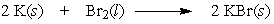
A) 2.00
B) 5.00
C) 10.0
D) 20.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
37
If 5.5 moles of pentane are burned according to the following equation:
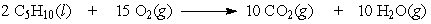 how many moles of oxygen are required?
how many moles of oxygen are required?
A) 41
B) 83
C) 11
D) 5.5
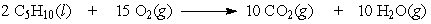 how many moles of oxygen are required?
how many moles of oxygen are required?A) 41
B) 83
C) 11
D) 5.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
38
According to the following equation, what mass of silver nitrate would be required to react with 0.500 grams potassium chloride? 
A) 0.500 g
B) 1.14 g
C) 85.0 g
D) 170 g

A) 0.500 g
B) 1.14 g
C) 85.0 g
D) 170 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
39
Methanol burns in air according to the equation below. How many grams of methanol can be burned by 10 grams of oxygen? 
A) 2 g
B) 7 g
C) 10 g
D) 20 g

A) 2 g
B) 7 g
C) 10 g
D) 20 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
40
Balance the equation below and determine the number of moles of oxygen required to completely burn 5.00 moles of propane. 
A) 5.00
B) 10.0
C) 15.0
D) 25.0

A) 5.00
B) 10.0
C) 15.0
D) 25.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
41
How many grams of oxygen (O2) are needed to completely react with 24.0 g of propane (C3H8) according to the equation below? 
A) 0.545 g
B) 2.73 g
C) 87.2 g
D) 28.8 g

A) 0.545 g
B) 2.73 g
C) 87.2 g
D) 28.8 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
42
In the following reaction, how many grams of water are needed to completely hydrolyze 30.8 g of the ester? 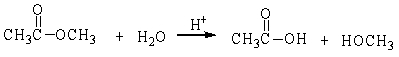
A) 7.48 g
B) 0.416 g
C) 30.8 g
D) 11.3 g

A) 7.48 g
B) 0.416 g
C) 30.8 g
D) 11.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
43
A test used by geologists to determine if the carbonate ion is present in rock can be performed by dripping a hydrochloric acid solution on the sample and looking for the bubbling of carbon dioxide. How many moles of carbon dioxide are produced by the reaction of 0.75 moles of limestone, CaCO3? 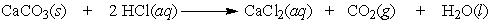
A) 2.35 moles
B) 2 moles
C) 1 mole
D) 0.75 moles
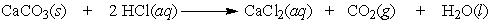
A) 2.35 moles
B) 2 moles
C) 1 mole
D) 0.75 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
44
The percent yield of a reaction is determined by dividing ___ in grams or moles by the ___ in grams or moles times 100%
A) theoretical yield, actual yield
B) actual yield, theoretical yield
C) amount of reactant, amount of products
D) amount of products, amount of reactants
A) theoretical yield, actual yield
B) actual yield, theoretical yield
C) amount of reactant, amount of products
D) amount of products, amount of reactants

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
45
If 2.00 grams of potassium are allowed to react with 2.00 grams of bromine by the following equation, which reactant is the limiting reactant? 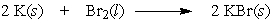
A) K
B) Br2
C) cannot be determined
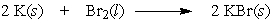
A) K
B) Br2
C) cannot be determined

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
46
A chemist runs a reaction to prepare bromobenzene, C6H5Br, from the reaction of benzene (C6H6) with bromine(Br2):
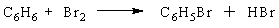 The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
A) 93%
B) 81%
C) 78 %
D) 45%
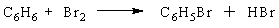 The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?A) 93%
B) 81%
C) 78 %
D) 45%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
47
If there is a "limiting reactant" in the hypothetical reaction,
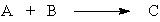 it is the substance
it is the substance
A) on the right that is produced in a limited amount.
B) on the left that is used to produce substance C on the right.
C) on the left that is used up first.
D) on the left that is limited by the amount of C produced.
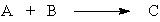 it is the substance
it is the substanceA) on the right that is produced in a limited amount.
B) on the left that is used to produce substance C on the right.
C) on the left that is used up first.
D) on the left that is limited by the amount of C produced.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
48
Sodium stearate is a soap that is produced from stearic acid, one of the fatty acids, by the reaction:
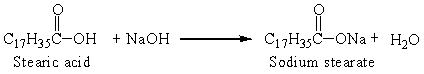 The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
A) 100%.
B) 93%.
C) 25%.
D) 1%.
 The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield was
The reaction that was set up with 0.25 g stearic acid and an excess of sodium hydroxide produced 0.25 grams soap. The percent yield wasA) 100%.
B) 93%.
C) 25%.
D) 1%.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
49
What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine? 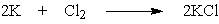
A) 5.2 g KCl
B) 4.9 g KCl
C) 9.9 g KCl
D) 10.1 g KCl
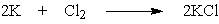
A) 5.2 g KCl
B) 4.9 g KCl
C) 9.9 g KCl
D) 10.1 g KCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
50
While this isn't a real reaction, we can say that carbonic acid, H2CO3, can be produced directly from the elements. Choose the statement that is correct when the given masses are supplied to react.
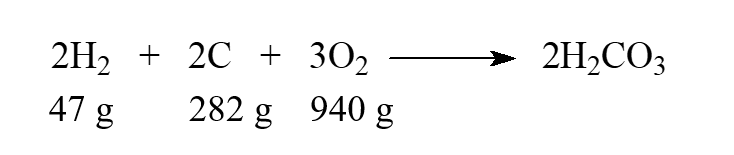
A) Hydrogen is the limiting reactant.
B) Carbon is the limiting reactant.
C) Oxygen is the limiting reactant.
D) There is no limiting reactant.
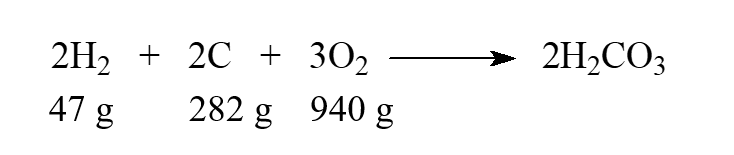
A) Hydrogen is the limiting reactant.
B) Carbon is the limiting reactant.
C) Oxygen is the limiting reactant.
D) There is no limiting reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
51
Two methanol molecules can react in the presence of sulfuric acid to produce dimethyl ether. Calculate the percent yield for this reaction that was run in a laboratory starting with 50 grams methanol and producing 26 grams dimethyl ether. 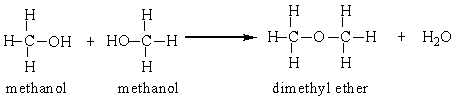
A) 52%
B) 57%
C) 72%
D) 200%

A) 52%
B) 57%
C) 72%
D) 200%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
52
Ethene, C2H4, burns in air according to the equation below. What is the theoretical yield (in grams) of CO2 when 3.24 g of C2H4 are reacted with 4.83 g of O2? 
A) 3.24 g
B) 9.72 g
C) 0.151 g
D) 4.43 g

A) 3.24 g
B) 9.72 g
C) 0.151 g
D) 4.43 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
53
Consider the burning of methane gas (CH4):
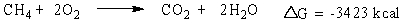 Which statement is true about the reaction?
Which statement is true about the reaction?
A) energy is absorbed and it is non-spontaneous
B) energy is released and it is non-spontaneous
C) energy is absorbed and it is spontaneous
D) energy is released and it is spontaneous
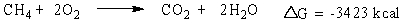 Which statement is true about the reaction?
Which statement is true about the reaction?A) energy is absorbed and it is non-spontaneous
B) energy is released and it is non-spontaneous
C) energy is absorbed and it is spontaneous
D) energy is released and it is spontaneous

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
54
___ lower the ___ of a reaction and therefore increase the reaction rate.
A) reactants, temperature
B) catalysts, activation energy
C) catalysts, concentration
D) activation energy, temperature
A) reactants, temperature
B) catalysts, activation energy
C) catalysts, concentration
D) activation energy, temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
55
The catalyst utilized by biological systems for hydrogenation reactions is ___.
A) platinum
B) hydrogen
C) an enzyme
D) a carbon-carbon bond
A) platinum
B) hydrogen
C) an enzyme
D) a carbon-carbon bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
56
Figures A and B below represent reaction energy diagrams. Diagram that represents an endothermic reaction is 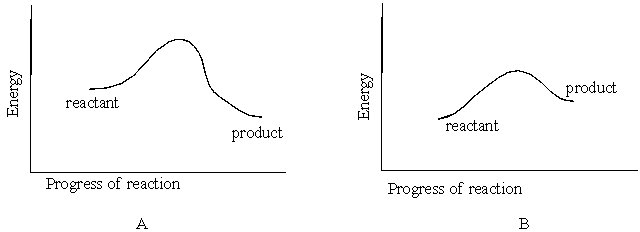
A) B
B) A
C) Both A and B
D) None of these choices

A) B
B) A
C) Both A and B
D) None of these choices

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
57
In the reaction energy diagram below, the activation energy is represented by: 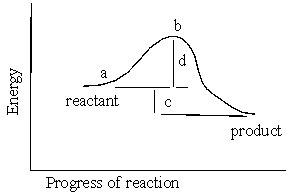
A) c
B) a
C) d
D) c.

A) c
B) a
C) d
D) c.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
58
Choose the comment that is appropriate for the reaction: 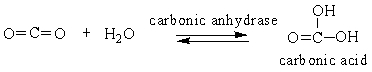
A) Carbonic anhydrase catalyzes the conversion of carbon dioxide into carbonic acid.
B) The reaction runs in forward direction only.
C) This reaction runs in backward direction only.
D) This is a reaction that only occurs in the laboratory.
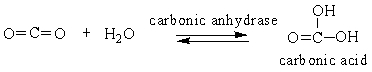
A) Carbonic anhydrase catalyzes the conversion of carbon dioxide into carbonic acid.
B) The reaction runs in forward direction only.
C) This reaction runs in backward direction only.
D) This is a reaction that only occurs in the laboratory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
59
The coefficients used to balance chemical equations can be interpreted in terms of atoms, moles, or grams.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
60
In a synthesis reaction, one compound breaks down to form elements or simpler compounds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
61
The reaction below can be classified as a double replacement reaction.



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
62
Platinum serves to catalyze some hydrogenation reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
63
In a hydrolysis reaction, water is used to split a molecule

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
64
An oxidation reaction can be defined as when an atom loses an oxygen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
65
An oxidizing agent is the reactant in an oxidation - reduction reaction that causes reduction of another reactant by providing electrons for the other reactant to accept.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
66
When G for a reaction is +286 kcal, the reaction is spontaneous.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
67
Compounds are formed from elements in a ___ reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
68
A synthesis reaction in which water is added to a double bond is called ___.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
69
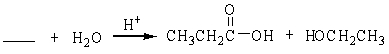

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
70
If the charge on an ion changes from 2+ to 4+, the process occurring is ___.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
71
For the following organic reactions, identify the reaction as an oxidation or reduction:
a)
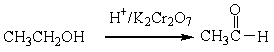
b)
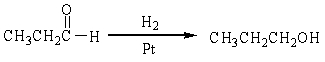
c)
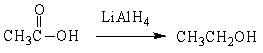
a)
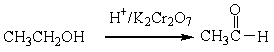
b)
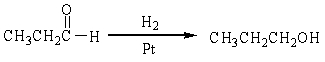
c)
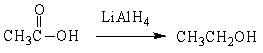

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
72
The measured amount of product obtained in any reaction is known as the ___.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
73
A reaction that releases heat is called ___.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
74
___ is the energy released or gained in a reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
75
Hydrolysis, hydration and dehydration reactions can take place in our cells with the aid of ____, which are proteins that catalyze reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
76
Aluminum carbonate was once the active ingredient in Rolaids . It reacts with stomach acid according to the following equation:
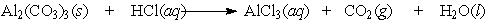
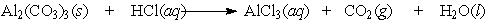

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
77
Balance the equation. Baking soda (NaHCO3) can be used as an antacid. It reacts with and neutralizes stomach acid (HCl). The equation for this reaction is as follows:
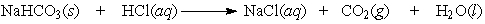
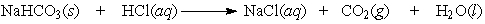

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
78
Balance the equation. Tums tablets are composed of the substance calcium carbonate. Two tablets have a combined mass of 5.0 grams. Calcium carbonate reacts with and neutralizes excess stomach acid (hydrochloric acid). The products of this reaction are: water, calcium chloride, and carbon dioxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
79
Write and balance the equation for the complete combustion of pentane (C5H12).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
80
Write and balance the equation for the complete combustion of cyclohexane (C6H12)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck








