Deck 16: Acids and Bases, a Molecular Look
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/104
العب
ملء الشاشة (f)
Deck 16: Acids and Bases, a Molecular Look
1
The conjugate acid of HPO42- is
A)H2PO4.
B)H3PO4.
C)PO43-.
D)PO42-.
E)H2PO4-.
A)H2PO4.
B)H3PO4.
C)PO43-.
D)PO42-.
E)H2PO4-.
H2PO4-.
2
The conjugate base of HPO42- is
A)H2PO4.
B)H3PO4.
C)PO43-.
D)PO42-.
E)H2PO4-.
A)H2PO4.
B)H3PO4.
C)PO43-.
D)PO42-.
E)H2PO4-.
PO43-.
3
The conjugate base of H2SO4 is
A)SO42-.
B)H3O+.
C)OH-.
D)HSO4-.
E)H2SO3.
A)SO42-.
B)H3O+.
C)OH-.
D)HSO4-.
E)H2SO3.
HSO4-.
4
The conjugate base of H2AsO4- is
A)H2AsO4
B)H3AsO4
C)HAsO4-
D)HAsO42-.
E)AsO43-.
A)H2AsO4
B)H3AsO4
C)HAsO4-
D)HAsO42-.
E)AsO43-.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
5
The conjugate acid of H2AsO4- is
A)H2AsO4
B)H3AsO4
C)HAsO4-
D)HAsO42-
E)AsO43-
A)H2AsO4
B)H3AsO4
C)HAsO4-
D)HAsO42-
E)AsO43-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which species is amphiprotic?
A)PO43-(aq)
B)HCl(g)
C)HSO4-(aq)
D)Cl-(aq)
E)CO32-(aq)
A)PO43-(aq)
B)HCl(g)
C)HSO4-(aq)
D)Cl-(aq)
E)CO32-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which species is amphoteric?
A)HNO3(aq)
B)Cl-(g)
C)CO32-(aq)
D)HSO3-(aq)
E)PO43-(aq)
A)HNO3(aq)
B)Cl-(g)
C)CO32-(aq)
D)HSO3-(aq)
E)PO43-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which species is amphiprotic?
A)AsO43-(aq)
B)HBr(aq)
C)HF(aq)
D)SO42-(aq)
E)HCO3-(aq)
A)AsO43-(aq)
B)HBr(aq)
C)HF(aq)
D)SO42-(aq)
E)HCO3-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which species does not have a conjugate base?
A)H2O
B)H3O+
C)NH4+
D)HCO3-
E)F-
A)H2O
B)H3O+
C)NH4+
D)HCO3-
E)F-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which species does not have a conjugate acid?
A)HCO2
B)H2O
C)HCl
D)OH-
E)NH2-
A)HCO2
B)H2O
C)HCl
D)OH-
E)NH2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which species has a conjugate base?
A)Cl-
B)SO42-
C)CN-
D)OH-
E)CO32-
A)Cl-
B)SO42-
C)CN-
D)OH-
E)CO32-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
12
In the reaction, HClO3 + N2H4  ClO3- + N2H5+, which species are a conjugate acid-base pair?
ClO3- + N2H5+, which species are a conjugate acid-base pair?
A)HClO3, N2H4
B)N2H4, ClO3-
C)HClO3, N2H5+
D)N2H4, N2H5+
E)ClO3-, N2H5+
 ClO3- + N2H5+, which species are a conjugate acid-base pair?
ClO3- + N2H5+, which species are a conjugate acid-base pair?A)HClO3, N2H4
B)N2H4, ClO3-
C)HClO3, N2H5+
D)N2H4, N2H5+
E)ClO3-, N2H5+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
13
In the reaction, HClO3 + N2H4  ClO3- + N2H5+, which two species are acids?
ClO3- + N2H5+, which two species are acids?
A)HClO3, N2H4
B)HClO3, ClO3-
C)HClO3, N2H5+
D)N2H4, N2H5+
E)ClO3-, N2H5+
 ClO3- + N2H5+, which two species are acids?
ClO3- + N2H5+, which two species are acids?A)HClO3, N2H4
B)HClO3, ClO3-
C)HClO3, N2H5+
D)N2H4, N2H5+
E)ClO3-, N2H5+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
14
In the reaction, HClO3 + N2H4  ClO3- + N2H5+, which two species are bases?
ClO3- + N2H5+, which two species are bases?
A)HClO3, N2H4
B)HClO3, ClO3-
C)HClO3, N2H5+
D)N2H4, N2H5+
E)ClO3-, N2H4
 ClO3- + N2H5+, which two species are bases?
ClO3- + N2H5+, which two species are bases?A)HClO3, N2H4
B)HClO3, ClO3-
C)HClO3, N2H5+
D)N2H4, N2H5+
E)ClO3-, N2H4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
15
In the reaction, H2PO4- + HAsO42-  HPO42- + H2AsO4-, which species are a conjugate acid-base pair?
HPO42- + H2AsO4-, which species are a conjugate acid-base pair?
A)H2PO4-, HAsO42-
B)H2PO4-, H2AsO4-
C)H2PO4-, HPO42-
D)HAsO42-, HPO42-
E)HPO42-, H2AsO4-
 HPO42- + H2AsO4-, which species are a conjugate acid-base pair?
HPO42- + H2AsO4-, which species are a conjugate acid-base pair?A)H2PO4-, HAsO42-
B)H2PO4-, H2AsO4-
C)H2PO4-, HPO42-
D)HAsO42-, HPO42-
E)HPO42-, H2AsO4-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
16
In the reaction, H2PO4- + HAsO42-  HPO42- + H2AsO4-, which two species are acids?
HPO42- + H2AsO4-, which two species are acids?
A)H2PO4-, HAsO42-
B)H2PO4-, H2AsO4-
C)H2PO4-, HPO42-
D)HAsO42-, HPO42-
E)HPO42-, H2AsO4-
 HPO42- + H2AsO4-, which two species are acids?
HPO42- + H2AsO4-, which two species are acids?A)H2PO4-, HAsO42-
B)H2PO4-, H2AsO4-
C)H2PO4-, HPO42-
D)HAsO42-, HPO42-
E)HPO42-, H2AsO4-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
17
In the reaction, H2PO4- + HAsO42-  HPO42- + H2AsO4-, which two species are bases?
HPO42- + H2AsO4-, which two species are bases?
A)H2PO4-, HAsO42-
B)H2PO4-, H2AsO4-
C)H2PO4-, HPO42-
D)HAsO42-, HPO42-
E)HPO42-, H2AsO4-
 HPO42- + H2AsO4-, which two species are bases?
HPO42- + H2AsO4-, which two species are bases?A)H2PO4-, HAsO42-
B)H2PO4-, H2AsO4-
C)H2PO4-, HPO42-
D)HAsO42-, HPO42-
E)HPO42-, H2AsO4-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
18
In the reaction, HSO4- + HS-  H2S + SO42-, which species are a conjugate acid-base pair?
H2S + SO42-, which species are a conjugate acid-base pair?
A)HSO4-, HS-
B)HSO4-, H2S
C)HS-, H2S
D)HS-, SO42-
E)H2S, SO42-
 H2S + SO42-, which species are a conjugate acid-base pair?
H2S + SO42-, which species are a conjugate acid-base pair?A)HSO4-, HS-
B)HSO4-, H2S
C)HS-, H2S
D)HS-, SO42-
E)H2S, SO42-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
19
In the reaction, HSO4- + HS-  H2S + SO42-, which two species are acids?
H2S + SO42-, which two species are acids?
A)HSO4-, HS-
B)HSO4-, H2S
C)HS-, H2S
D)HS-, SO42-
E)H2S, SO42-
 H2S + SO42-, which two species are acids?
H2S + SO42-, which two species are acids?A)HSO4-, HS-
B)HSO4-, H2S
C)HS-, H2S
D)HS-, SO42-
E)H2S, SO42-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
20
In the reaction, HSO4- + HS-  H2S + SO42-, which two species are bases?
H2S + SO42-, which two species are bases?
A)HSO4-, HS--
B)HSO4-, H2S
C)HS-, H2S
D)HS-, SO42-
E)H2S, SO42-
 H2S + SO42-, which two species are bases?
H2S + SO42-, which two species are bases?A)HSO4-, HS--
B)HSO4-, H2S
C)HS-, H2S
D)HS-, SO42-
E)H2S, SO42-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
21
In the reaction, HSO4- + CN-  HCN + SO42-, which species are a conjugate acid-base pair?
HCN + SO42-, which species are a conjugate acid-base pair?
A)HSO4-, CN-
B)CN-, HCN
C)CN-, SO42-
D)HCN, SO42-
E)HCN, HSO4-
 HCN + SO42-, which species are a conjugate acid-base pair?
HCN + SO42-, which species are a conjugate acid-base pair?A)HSO4-, CN-
B)CN-, HCN
C)CN-, SO42-
D)HCN, SO42-
E)HCN, HSO4-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
22
In the reaction, HSO4- + CN-  HCN + SO42-, which two species are acids?
HCN + SO42-, which two species are acids?
A)HSO4-, CN-
B)HSO4-, HCN
C)CN-, HCN
D)CN-, SO42-
E)SO42-, HCN
 HCN + SO42-, which two species are acids?
HCN + SO42-, which two species are acids?A)HSO4-, CN-
B)HSO4-, HCN
C)CN-, HCN
D)CN-, SO42-
E)SO42-, HCN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
23
In the reaction, HSO4- + CN-  HCN + SO42-, which two species are both bases?
HCN + SO42-, which two species are both bases?
A)HSO4-, CN-
B)HSO4-, HCN
C)SO42-, HCN
D)CN-, HCN
E)CN-, SO42-
 HCN + SO42-, which two species are both bases?
HCN + SO42-, which two species are both bases?A)HSO4-, CN-
B)HSO4-, HCN
C)SO42-, HCN
D)CN-, HCN
E)CN-, SO42-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
24
For the system NH2OH + CH3NH3+  CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the strongest acid in the system?
CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the strongest acid in the system?
A)NH2OH
B)CH3NH3+
C)CH3NH2
D)NH3OH+
E)NH2OH and CH3NH3+ are equal in acid strength, and are the strongest acids in the system.
 CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the strongest acid in the system?
CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the strongest acid in the system?A)NH2OH
B)CH3NH3+
C)CH3NH2
D)NH3OH+
E)NH2OH and CH3NH3+ are equal in acid strength, and are the strongest acids in the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
25
For the system HC6H5O + C4H7O2-  C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest acid in the system?
C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest acid in the system?
A)HC6H5O
B)C4H7O2-
C)C6H5O-
D)HC4H7O2
E)HC4H7O2 and C4H7O2- are equal in acid strength, and are the strongest acids in the system
 C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest acid in the system?
C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest acid in the system?A)HC6H5O
B)C4H7O2-
C)C6H5O-
D)HC4H7O2
E)HC4H7O2 and C4H7O2- are equal in acid strength, and are the strongest acids in the system

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
26
For the system NH2OH + CH3NH3+  CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the weakest base in the system?
CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the weakest base in the system?
A)NH2OH
B)CH3NH3+
C)CH3NH2
D)NH3OH+
E)CH3NH2 and NH3OH+ are equal in base strength and are the weakest bases in the system
 CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the weakest base in the system?
CH3NH2 + NH3OH+ the position of the equilibrium lies to the left. Which is the weakest base in the system?A)NH2OH
B)CH3NH3+
C)CH3NH2
D)NH3OH+
E)CH3NH2 and NH3OH+ are equal in base strength and are the weakest bases in the system

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
27
For the system HC6H5O + C4H7O2-  C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest base in the system?
C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest base in the system?
A)HC6H5O
B)C4H7O2-
C)C6H5O-
D)HC4H7O2
E)HC4H7O2 and C4H7O2- are equal in acid strength, and are the strongest acids in the system
 C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest base in the system?
C6H5O- + HC4H7O2 the position of the equilibrium lies to the left. Which is the strongest base in the system?A)HC6H5O
B)C4H7O2-
C)C6H5O-
D)HC4H7O2
E)HC4H7O2 and C4H7O2- are equal in acid strength, and are the strongest acids in the system

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
28
Given the following substances, listed in order of increasing acid strength,HOCl(aq)< HC2H3O2(aq)< HC2O4-(aq)< HOCN(aq)< HNO2(aq)< HCl(aq)Which species listed below is the strongest base?
A)Cl-(aq)
B)OCl-(aq)
C)H2C2O4(aq)
D)NO2-(aq)
E)OCN-(aq)
A)Cl-(aq)
B)OCl-(aq)
C)H2C2O4(aq)
D)NO2-(aq)
E)OCN-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
29
Given the following substances, listed in order of increasing acid strength,HOCl(aq)< HC2H3O2(aq)< HC2O4-(aq)< HOCN(aq)< HNO2(aq)< HCl(aq)Which species listed below is the weakest base?
A)C2H3O2-(aq)
B)OCl-(aq)
C)H2C2O4(aq)
D)Cl-(aq)
E)OCN-(aq)
A)C2H3O2-(aq)
B)OCl-(aq)
C)H2C2O4(aq)
D)Cl-(aq)
E)OCN-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
30
Given the following substances, listed in order of increasing acid strength,HOCl(aq)< HC2H3O2(aq)< HC2O4-(aq)< HOCN(aq)< HNO2(aq)< HCl(aq)Which species listed below is the strongest base?
A)Cl-(aq)
B)HOCl(aq)
C)C2O42-(aq)
D)NO2-(aq)
E)OCN-(aq)
A)Cl-(aq)
B)HOCl(aq)
C)C2O42-(aq)
D)NO2-(aq)
E)OCN-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
31
Given the following substances in order of increasing acid strength,HOCl(aq)< HC2H3O2(aq)< HC2O4-(aq)< HOCN(aq)< HNO2(aq)< HCl(aq)Which species listed below is the weakest base of that set?
A)C2H3O2-(aq)
B)OCl-(aq)
C)HC2O4-(aq)
D)HNO2(aq)
E)OCN-(aq)
A)C2H3O2-(aq)
B)OCl-(aq)
C)HC2O4-(aq)
D)HNO2(aq)
E)OCN-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
32
Given that X is the same atom for each of the following, which is the strongest oxyacid?
A)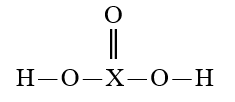
B)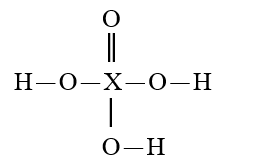
C)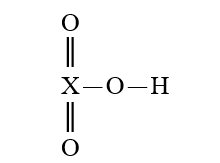
D)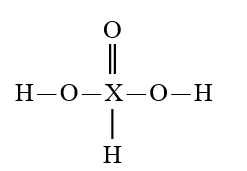
E)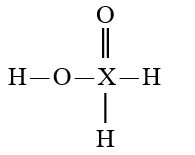
A)
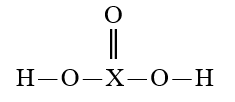
B)
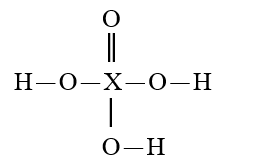
C)
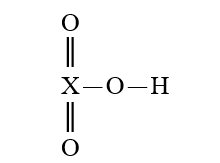
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
33
Given that X is the same atom for each of the following, which is the strongest oxyacid?
A)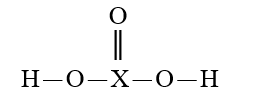
B)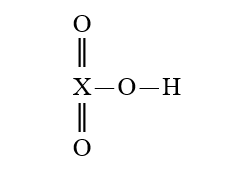
C)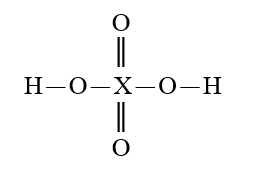
D)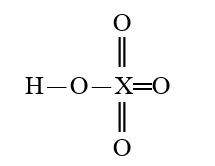
E)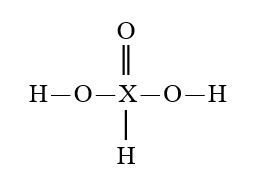
A)
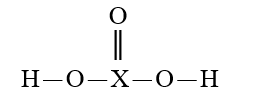
B)
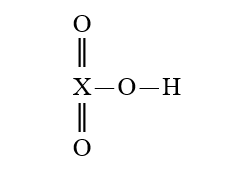
C)
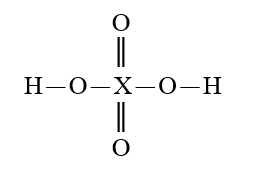
D)
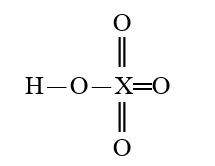
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
34
Given that X is the same atom for each of the following, which is the weakest oxoacid?
A)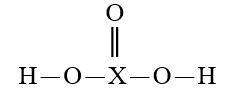
B)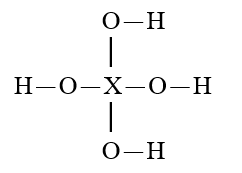
C)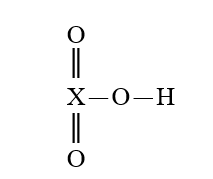
D)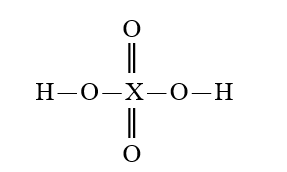
E)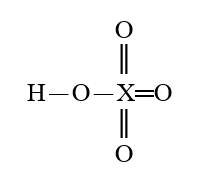
A)
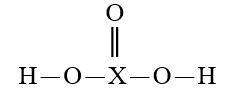
B)
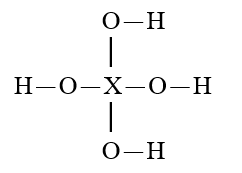
C)
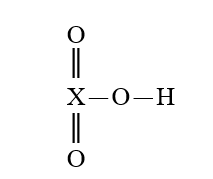
D)
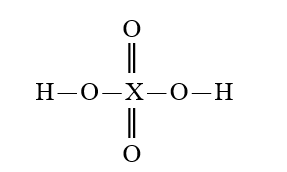
E)
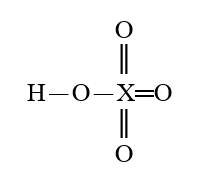

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
35
Given that X is the same atom for each of the following, which is the strongest oxoacid?
A)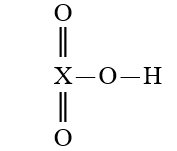
B)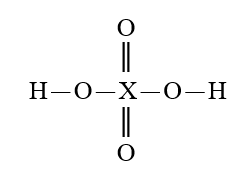
C)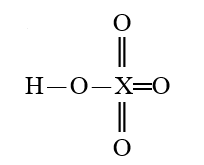
D)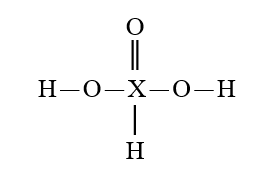
E)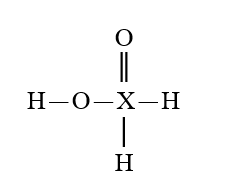
A)
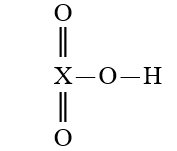
B)
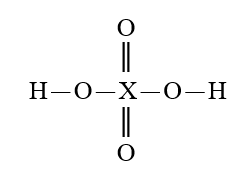
C)
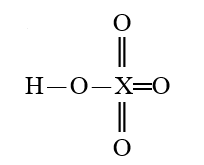
D)
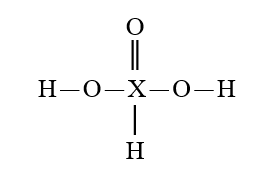
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which species would be expected to be the most acidic?
A)HClO
B)HClO2
C)HClO3
D)HClO4
E)Cl-
A)HClO
B)HClO2
C)HClO3
D)HClO4
E)Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which species is the weakest acid?
A)HBr
B)HCl
C)HF
D)HI
E)H2Se
A)HBr
B)HCl
C)HF
D)HI
E)H2Se

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which species is the strongest organic acid?
A)CCl3-COOH
B)CH3-COOH
C)CH2Cl-COOH
D)CBr3-COOH
E)CBrCl2-COOH
A)CCl3-COOH
B)CH3-COOH
C)CH2Cl-COOH
D)CBr3-COOH
E)CBrCl2-COOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
39
For the reaction, CO2 + H2O  H2CO3, which of the following statements is incorrect?
H2CO3, which of the following statements is incorrect?
A)CO2 is a Brønsted-Lowry acid and H2O is a Brønsted-Lowry base.
B)H2CO3 forms a coordinate covalent bond by a Lewis acid-base reaction.
C)CO2 is a Lewis acid and H2O is a Lewis base.
D)The lone pairs in the H2O molecule are used to form a new bond with the carbon in the CO2 molecule.
E)Both A and D
 H2CO3, which of the following statements is incorrect?
H2CO3, which of the following statements is incorrect?A)CO2 is a Brønsted-Lowry acid and H2O is a Brønsted-Lowry base.
B)H2CO3 forms a coordinate covalent bond by a Lewis acid-base reaction.
C)CO2 is a Lewis acid and H2O is a Lewis base.
D)The lone pairs in the H2O molecule are used to form a new bond with the carbon in the CO2 molecule.
E)Both A and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
40
NH3 can react with BF3, forming NH3-BF3. In this reaction,
A)the NH3 is a Brønsted-Lowry base, accepting a proton from the BF3 molecule.
B)the NH3 is a Lewis base, donating a proton to the BF3 molecule.
C)the NH3 is a Brønsted-Lowry acid, donating a proton to the BF3 molecule.
D)the BF3 molecule is a Lewis acid, accepting an electron pair from the NH3 molecule to form a coordinate covalent bond.
E)the BF3 molecule is a Lewis base, donating an electron pair to the NH3 molecule to form a coordinate covalent bond.
A)the NH3 is a Brønsted-Lowry base, accepting a proton from the BF3 molecule.
B)the NH3 is a Lewis base, donating a proton to the BF3 molecule.
C)the NH3 is a Brønsted-Lowry acid, donating a proton to the BF3 molecule.
D)the BF3 molecule is a Lewis acid, accepting an electron pair from the NH3 molecule to form a coordinate covalent bond.
E)the BF3 molecule is a Lewis base, donating an electron pair to the NH3 molecule to form a coordinate covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
41
SO2 can react with OH-, forming HSO3-. In this reaction,
A)the SO2 is a Brønsted-Lowry acid, accepting a proton from the OH- ion.
B)the SO2 is a Lewis acid, accepting a proton from the OH- ion.
C)the OH- ion is a Brønsted-Lowry base, donating a proton to the SO2 molecule.
D)the OH- ion is a Lewis base, donating an electron pair to the SO2 molecule to form a coordinate covalent bond.
E)the OH- ion is a Lewis acid, accepting an electron pair from the SO2 molecule to form a coordinate covalent bond.
A)the SO2 is a Brønsted-Lowry acid, accepting a proton from the OH- ion.
B)the SO2 is a Lewis acid, accepting a proton from the OH- ion.
C)the OH- ion is a Brønsted-Lowry base, donating a proton to the SO2 molecule.
D)the OH- ion is a Lewis base, donating an electron pair to the SO2 molecule to form a coordinate covalent bond.
E)the OH- ion is a Lewis acid, accepting an electron pair from the SO2 molecule to form a coordinate covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
42
The F- ion can react with BF3, forming the BF4- anion. In this reaction,
A)the F- ion is a Brønsted-Lowry base, accepting a proton from the BF3 molecule.
B)the F- ion is a Lewis base, donating a proton to the BF3 molecule.
C)the F- ion is a Brønsted-Lowry acid, donating a proton to the BF3 molecule.
D)the BF3 molecule acts as a Lewis acid, accepting an electron pair from the F- ion to form a coordinate covalent bond.
E)the BF3 molecule acts as a Lewis base, donating an electron pair to the F- ion to form a coordinate covalent bond.
A)the F- ion is a Brønsted-Lowry base, accepting a proton from the BF3 molecule.
B)the F- ion is a Lewis base, donating a proton to the BF3 molecule.
C)the F- ion is a Brønsted-Lowry acid, donating a proton to the BF3 molecule.
D)the BF3 molecule acts as a Lewis acid, accepting an electron pair from the F- ion to form a coordinate covalent bond.
E)the BF3 molecule acts as a Lewis base, donating an electron pair to the F- ion to form a coordinate covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
43
CO2 can react with OH-, forming HCO3-. In this reaction,
A)the CO2 is a Brønsted-Lowry acid, accepting a proton from the OH- ion.
B)the CO2 is a Lewis acid, accepting a proton from the OH- ion.
C)the OH- ion is a Brønsted-Lowry base, donating a proton to the CO2 molecule.
D)the OH- ion is a Lewis acid, accepting an electron pair from the CO2 molecule to form a coordinate covalent bond.
E)the OH- ion is a Lewis base, donating an electron pair to the CO2 molecule to form a coordinate covalent bond.
A)the CO2 is a Brønsted-Lowry acid, accepting a proton from the OH- ion.
B)the CO2 is a Lewis acid, accepting a proton from the OH- ion.
C)the OH- ion is a Brønsted-Lowry base, donating a proton to the CO2 molecule.
D)the OH- ion is a Lewis acid, accepting an electron pair from the CO2 molecule to form a coordinate covalent bond.
E)the OH- ion is a Lewis base, donating an electron pair to the CO2 molecule to form a coordinate covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which pair of reactants will yield H2SO4 as one of its products?
A)SO2 + OH-
B)SO3 + OH-
C)SO2 + H2O
D)SO42- + OH-
E)SO3 + H2O
A)SO2 + OH-
B)SO3 + OH-
C)SO2 + H2O
D)SO42- + OH-
E)SO3 + H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which pair of reactants will yield H2SO3 as one of its products?
A)SO2 + OH-
B)SO3 + OH-
C)SO2 + H2O
D)SO42- + OH-
E)SO3 + H2O
A)SO2 + OH-
B)SO3 + OH-
C)SO2 + H2O
D)SO42- + OH-
E)SO3 + H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which 0.1 M solution would be the most acidic?
A)Al(NO3)3 (aq)
B)Ca(NO3)2 (aq)
C)KNO3 (aq)
D)Mg(NO3)2 (aq)
E)Zn(NO3)2 (aq)
A)Al(NO3)3 (aq)
B)Ca(NO3)2 (aq)
C)KNO3 (aq)
D)Mg(NO3)2 (aq)
E)Zn(NO3)2 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which 0.1 M solution would be the most acidic?
A)ZnCl2 (aq)
B)CdCl2 (aq)
C)FeCl3 (aq)
D)AuCl3 (aq)
E)NaCl (aq)
A)ZnCl2 (aq)
B)CdCl2 (aq)
C)FeCl3 (aq)
D)AuCl3 (aq)
E)NaCl (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which 0.1 M solution would be the least acidic?
A)ZnCl2 (aq)
B)CdCl2 (aq)
C)FeCl3 (aq)
D)NiCl2 (aq)
E)CuCl2 (aq)
A)ZnCl2 (aq)
B)CdCl2 (aq)
C)FeCl3 (aq)
D)NiCl2 (aq)
E)CuCl2 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
49
Five solutions were prepared by dissolving 0.00100 mole of each of the following compounds in enough water to make 1.000 liter of solution. Which one of these compounds would make the most acidic solution?
A)CO2
B)P4O6
C)P4O10
D)SO2
E)SO3
A)CO2
B)P4O6
C)P4O10
D)SO2
E)SO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following would form an acidic solution when added to water?
A)CO2 (g)
B)Na2O (s)
C)CaO (s)
D)H2O (g)
E)None of these
A)CO2 (g)
B)Na2O (s)
C)CaO (s)
D)H2O (g)
E)None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following would form an acidic solution when added to water?
A)SO2 (g)
B)Na2O (s)
C)MgO (s)
D)H2O (g)
E)None of these
A)SO2 (g)
B)Na2O (s)
C)MgO (s)
D)H2O (g)
E)None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following would form a basic solution when added to water?
A)CO2 (g)
B)Na2O (s)
C)SO3 (g)
D)H2O (g)
E)P4O6 (g)
A)CO2 (g)
B)Na2O (s)
C)SO3 (g)
D)H2O (g)
E)P4O6 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following would form a basic solution when added to water?
A)NO2 (g)
B)K2O (s)
C)SO3 (g)
D)H2O (g)
E)P4O6 (g)
A)NO2 (g)
B)K2O (s)
C)SO3 (g)
D)H2O (g)
E)P4O6 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which solution should be the least acidic?
A)Al(NO3)3 (aq)
B)Ga(NO3)3 (aq)
C)AgNO3 (aq)
D)Cu(NO3)2 (aq)
E)Zn(NO3)2 (aq)
A)Al(NO3)3 (aq)
B)Ga(NO3)3 (aq)
C)AgNO3 (aq)
D)Cu(NO3)2 (aq)
E)Zn(NO3)2 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which solution should be the most acidic?
A)Zn(NO3)2 (aq)
B)Cd(NO3)2 (aq)
C)Ga(NO3)3 (aq)
D)Hg(NO3)2 (aq)
E)Al(NO3)3 (aq)
A)Zn(NO3)2 (aq)
B)Cd(NO3)2 (aq)
C)Ga(NO3)3 (aq)
D)Hg(NO3)2 (aq)
E)Al(NO3)3 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which solution should be the least acidic?
A)Al(NO3)3 (aq)
B)Cd(NO3)2 (aq)
C)Ga(NO3)3 (aq)
D)KNO3 (aq)
E)Zn(NO3)2 (aq)
A)Al(NO3)3 (aq)
B)Cd(NO3)2 (aq)
C)Ga(NO3)3 (aq)
D)KNO3 (aq)
E)Zn(NO3)2 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
57
Five solutions were prepared by dissolving 0.00100 mole of each of the following in enough water to make 1.000 liter of solution. Which one of these compounds would make the most acidic solution?
A)CO2
B)P4O6
C)P4O10
D)SO2
E)SO3
A)CO2
B)P4O6
C)P4O10
D)SO2
E)SO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
58
Five solutions were prepared by dissolving 0.00100 mole of each of the following in enough water to make 1.000 liter of solution. Which one of these compounds would make the most acidic solution?
A)Na2O
B)BaO
C)Ga2O3
D)Al2O3
E)P4O6
A)Na2O
B)BaO
C)Ga2O3
D)Al2O3
E)P4O6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
59
Five solutions were prepared by dissolving 0.00100 mole of each of the following in enough water to make 1.000 liter of solution. Which one of these compounds would make the most acidic solution?
A)Na2O
B)BaO
C)Ga2O3
D)Al2O3
E)P4O6
A)Na2O
B)BaO
C)Ga2O3
D)Al2O3
E)P4O6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
60
The step in the sol-gel process that forms oxygen bridges while forming water molecules can be best classified as
A)an acid-base reaction.
B)sintering.
C)an evaporation process.
D)annealing.
E)metallurgy.
A)an acid-base reaction.
B)sintering.
C)an evaporation process.
D)annealing.
E)metallurgy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
61
Ceramic material made by the sol-gel process involve the reaction of
A)water and acids.
B)ethanol and water.
C)water and metal oxides.
D)acids and metal alkoxides.
E)water and metal alkoxides.
A)water and acids.
B)ethanol and water.
C)water and metal oxides.
D)acids and metal alkoxides.
E)water and metal alkoxides.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the formula for the conjugate base of NH3(aq)?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the formula for the conjugate acid of the OH-(aq)ion?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the formula for the conjugate acid of the HCO3-(aq)ion?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the formula for the conjugate base of the HCO3-(aq)ion?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the formula for the conjugate base of HNO3(aq)?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
67
In the reaction, HClO3(aq)+ N2H4(aq)  ClO3-(aq)+ N2H5+(aq), list the species that are conjugate acid-base pairs.
ClO3-(aq)+ N2H5+(aq), list the species that are conjugate acid-base pairs.
 ClO3-(aq)+ N2H5+(aq), list the species that are conjugate acid-base pairs.
ClO3-(aq)+ N2H5+(aq), list the species that are conjugate acid-base pairs.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
68
In the reaction, HSO4-(aq)+ OH-(aq)  SO42-(aq)+ H2O(l), list the species that are conjugate acid-base pairs.
SO42-(aq)+ H2O(l), list the species that are conjugate acid-base pairs.
 SO42-(aq)+ H2O(l), list the species that are conjugate acid-base pairs.
SO42-(aq)+ H2O(l), list the species that are conjugate acid-base pairs.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
69
Explain why the anion HCO3- can be classified as an amphoteric species.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
70
The strongest base that can exist in aqueous solution is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
71
The strongest acid that can exist in aqueous solution is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
72
For the system NH2OH(aq)+ CH3NH3+(aq)  CH3NH2(aq)+ NH3OH+(aq)the position of the equilibrium lies to the left. What is the strongest acid in this reaction?
CH3NH2(aq)+ NH3OH+(aq)the position of the equilibrium lies to the left. What is the strongest acid in this reaction?
 CH3NH2(aq)+ NH3OH+(aq)the position of the equilibrium lies to the left. What is the strongest acid in this reaction?
CH3NH2(aq)+ NH3OH+(aq)the position of the equilibrium lies to the left. What is the strongest acid in this reaction?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
73
For the system NH2OH(aq)+ CH3NH3+(aq)  CH3NH2(aq)+ NH3OH+(aq)the position of equilibrium lies to the left. What is the strongest base in this reaction?
CH3NH2(aq)+ NH3OH+(aq)the position of equilibrium lies to the left. What is the strongest base in this reaction?
 CH3NH2(aq)+ NH3OH+(aq)the position of equilibrium lies to the left. What is the strongest base in this reaction?
CH3NH2(aq)+ NH3OH+(aq)the position of equilibrium lies to the left. What is the strongest base in this reaction?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
74
For the system H3PO4(aq)+ COOH-(aq)  HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest acid in this reaction?
HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest acid in this reaction?
 HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest acid in this reaction?
HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest acid in this reaction?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
75
For the system H3PO4(aq)+ COOH-(aq)  HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest base in this reaction?
HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest base in this reaction?
 HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest base in this reaction?
HCOOH(aq)+ H2PO4-(aq)the position of equilibrium lies to the right. What is the strongest base in this reaction?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
76
Three oxoacids with the formulas shown are listed in order of decreasing acid strength:HZO3 > HYO3 > HXO3What is the formula for the strongest conjugate base of these acids?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
77
Three binary acids with the formulas shown are listed in order of decreasing acid strength:HZ > HY > HX What is the formula for the strongest conjugate base of these acids?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which one of the two species, H2Se or H3As, is the stronger Brønsted-Lowry acid?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which one of the two species, HF(aq)or HI(aq), is the weaker Brønsted-Lowry acid?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which one of the two species, H2S or HBr, is the stronger Brønsted-Lowry acid?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 104 في هذه المجموعة.
فتح الحزمة
k this deck








