Deck 13: Kinetics: Mechanisms and Rates of Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/77
العب
ملء الشاشة (f)
Deck 13: Kinetics: Mechanisms and Rates of Reactions
1
Explain the concepts of a mechanism and a rate-determining step in a chemical reaction.
A reaction mechanism is the exact molecular pathway that starting materials follow on their way to becoming products. The overall reaction is the sum of the elementary steps in the mechanism. The rate-determining step is the slowest step in the reaction mechanism. The overall reaction cannot go faster than the rate-determining step.
2
Determine the rate of a reaction based on the rate of change of concentration of a reactant or a product.
The reaction rate can be expressed using the rate of change of concentration of any of the reagents.
3
Determine the rate law, given the mechanism and knowledge of the relative rates of steps of a reaction.
A reaction rate depends on the concentrations of the reactants as expressed by the rate law for that reaction. The order of a reactant often differs from its stoichiometric coefficient.
4
Determine rate laws from concentration versus time data.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
5
Show that the mechanism and rate law are closely related.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
6
Explain and quantify the effects of temperature on a reaction rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
7
Explain the mechanisms by which catalysts function.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following are bimolecular processes?
I.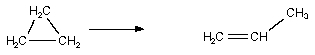
II. NO ONNO
III. H2C = CHCH3 + H2SO4 CH3CH(OSO2(OH))CH3
IV.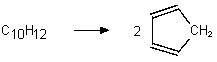
V. H2 2 H•
A) I and V
B) I, IV and V
C) II only
D) II and III
E) II, III and V
I.
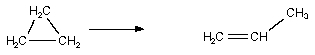
II. NO ONNO
III. H2C = CHCH3 + H2SO4 CH3CH(OSO2(OH))CH3
IV.
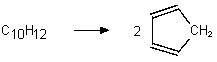
V. H2 2 H•
A) I and V
B) I, IV and V
C) II only
D) II and III
E) II, III and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following is an elementary reaction?
A) OH- + H3O+ H2O
B) 4NO2 + O2 2N2O5
C) CO + 2H2 + CH4 C2H6 + H2O
D) N2 + 3H2 NH3
E) 2NO + 2H2 N2 + 2H2O
A) OH- + H3O+ H2O
B) 4NO2 + O2 2N2O5
C) CO + 2H2 + CH4 C2H6 + H2O
D) N2 + 3H2 NH3
E) 2NO + 2H2 N2 + 2H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
10
The formation of chlorocarbon solvents such as CH2Cl2 proceeds through the reaction of chlorine with methane. Which of the following is a unimolecular reaction in the mechanism?
A) CH4 + Cl• CH3• + HCl
B) H2 2 H•
C) CH3• + Cl• CH3Cl
D) Cl2 2 Cl•
E) CH2Cl• + Cl• CH2Cl2
A) CH4 + Cl• CH3• + HCl
B) H2 2 H•
C) CH3• + Cl• CH3Cl
D) Cl2 2 Cl•
E) CH2Cl• + Cl• CH2Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
11
It has been suggested that the decomposition of NO2 occurs via the following mechanism:
NO2 NO + O (Rxn I)
O + NO2 2NO + O2 (Rxn II)
Predict the rate determining step.
A) Rxn II; this reaction is bimolecular, bimolecular reactions are always slower than unimolecular reactions.
B) Rxn I; unimolecular reactions are always slower than bimolecular reactions.
C) Rxn I; O is highly reactive and thus Rxn II will be very fast.
D) Rxn II; NO2 is highly reactive and thus Rxn I will be very fast.
E) Rxn I and II are both elementary reactions and will proceed at equal rates.
NO2 NO + O (Rxn I)
O + NO2 2NO + O2 (Rxn II)
Predict the rate determining step.
A) Rxn II; this reaction is bimolecular, bimolecular reactions are always slower than unimolecular reactions.
B) Rxn I; unimolecular reactions are always slower than bimolecular reactions.
C) Rxn I; O is highly reactive and thus Rxn II will be very fast.
D) Rxn II; NO2 is highly reactive and thus Rxn I will be very fast.
E) Rxn I and II are both elementary reactions and will proceed at equal rates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
12
You are running late for a basketball game on your campus and you are thinking about what will be the rate determining step for attending the basketball game? Which of the following will be your rate determining step?
A) purchasing a ticket
B) locating a seat in the stands
C) walking/driving to the game
D) waiting for the players to arrive
E) giving your ticket to the doorman
A) purchasing a ticket
B) locating a seat in the stands
C) walking/driving to the game
D) waiting for the players to arrive
E) giving your ticket to the doorman

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
13
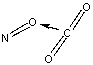
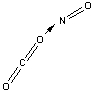
Which of the following sketches shows the proper orientation of molecules for CO2 reacting with NO to make NO2 and CO?
A)
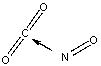
B)
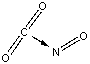
C)
D)
E)
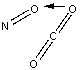
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
14
The synthesis of nitrogen monoxide proceeds by the reaction of ammonia with oxygen as shown in the following unbalanced reaction:
NH3(g) + O2(g) NO(g) + H2O(g)If O2 is being consumed at a rate of 32 mole/sec, what is the rate of NO production?
A) 38. mole/sec
B) 1.9 x 103 mole/minute
C) 15 x 103 mole/minute
D) 32 mole/minute
E) 1.4 x 105 mole/hour
NH3(g) + O2(g) NO(g) + H2O(g)If O2 is being consumed at a rate of 32 mole/sec, what is the rate of NO production?
A) 38. mole/sec
B) 1.9 x 103 mole/minute
C) 15 x 103 mole/minute
D) 32 mole/minute
E) 1.4 x 105 mole/hour

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
15
Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the reaction rate expressed in terms of changing H3O+ concentration?
A) Reaction rate =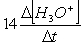
B) Reaction rate = -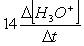
C) Reaction rate = -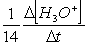
D) Reaction rate =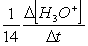
A) Reaction rate =
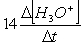
B) Reaction rate = -
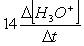
C) Reaction rate = -
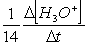
D) Reaction rate =
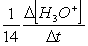

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
16
Consider the aqueous phase reaction between hydrogen gas and liquid bromine:H2(g) + Br2(g) 2HBr(g)Which of the following expressions accurately express the rate of the above reaction?
I. Reaction rate =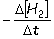
II. Reaction rate =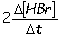
III. Reaction rate =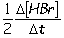
IV. Reaction rate =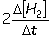
A) I and III
B) I and II
C) II and IV
D) III and IV
E) I only
I. Reaction rate =
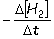
II. Reaction rate =
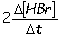
III. Reaction rate =
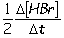
IV. Reaction rate =
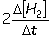
A) I and III
B) I and II
C) II and IV
D) III and IV
E) I only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
17
Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the rate of increase of Cr3+ concentration expressed in terms of changing H3O+ concentration?
A)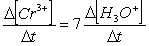
B)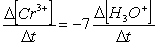
C)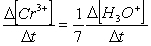
D)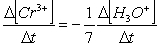
A)
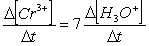
B)
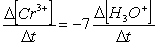
C)
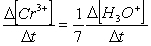
D)
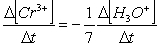

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
18
Cyclohexane is manufactured from the reaction of benzene with hydrogen:
C6H6(g) + 3 H2(g) C6H12(g)
If the initial concentration of hydrogen was 1.5 M and 5 minutes later the hydrogen concentration is 0.34 M, what is the average rate of disappearance of hydrogen?
A) 0.30 M/min
B) 0.20 M/min
C) 1.16 M/min
D) 0.23 M/min
E) 0.68 M/min
C6H6(g) + 3 H2(g) C6H12(g)
If the initial concentration of hydrogen was 1.5 M and 5 minutes later the hydrogen concentration is 0.34 M, what is the average rate of disappearance of hydrogen?
A) 0.30 M/min
B) 0.20 M/min
C) 1.16 M/min
D) 0.23 M/min
E) 0.68 M/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
19
Cyclohexane is manufactured from the reaction of benzene with hydrogen:
C6H6(g) + 3 H2(g) C6H12(g)
If the initial concentration of hydrogen was 1.5 M and 5 minutes later the hydrogen concentration is 0.34 M, what is the average rate of appearance of cyclohexane?
A) 0.39 M/min
B) 7.7 x 10-2 M/min
C) 4.6 M/s
D) 3.9 x 10- 2 M/hr
E) 2.3 M/min
C6H6(g) + 3 H2(g) C6H12(g)
If the initial concentration of hydrogen was 1.5 M and 5 minutes later the hydrogen concentration is 0.34 M, what is the average rate of appearance of cyclohexane?
A) 0.39 M/min
B) 7.7 x 10-2 M/min
C) 4.6 M/s
D) 3.9 x 10- 2 M/hr
E) 2.3 M/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
20
NO2 decomposes to form NO and O2. The concentration of NO2 is monitored and found to be 2.1x10-2 M after 15 seconds and 1.8 x10-2 M after 20 seconds. What is the average rate of appearance of O2 over this time period?
A) 6.0x10-4 M/s
B) 1.2x10-3 M/s
C) 3.0x10-4 M/s
D) 3.0x10-3 M/s
E) 1.5x10-4 M/s
A) 6.0x10-4 M/s
B) 1.2x10-3 M/s
C) 3.0x10-4 M/s
D) 3.0x10-3 M/s
E) 1.5x10-4 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
21
Why does the rate of a reaction generally slow with time?
A) The reaction mixture gets warmer.
B) The number of product molecules decreases.
C) The number of collisions of reactants decreases.
D) The number of product molecules increases.
E) The temperature of the reaction vessel decreases.
A) The reaction mixture gets warmer.
B) The number of product molecules decreases.
C) The number of collisions of reactants decreases.
D) The number of product molecules increases.
E) The temperature of the reaction vessel decreases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
22
The rate of a reaction
A) increases with the concentration of reactants.
B) decreases with the concentration of reactants.
C) is independent of concentration.
D) is a function of reaction stoichiometry.
E) cannot be determined based on the balanced chemical reaction.
A) increases with the concentration of reactants.
B) decreases with the concentration of reactants.
C) is independent of concentration.
D) is a function of reaction stoichiometry.
E) cannot be determined based on the balanced chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
23
For the reaction I-(aq) + OCl-(aq) IO-(aq) + Cl-(aq) in basic solution, it was found that ![<strong>For the reaction I<sup>-</sup>(aq) + OCl<sup>-</sup>(aq) \rarr IO<sup>-</sup>(aq) + Cl<sup>-</sup>(aq) in basic solution, it was found that </strong> A) This reaction is first order overall. B) This reaction is third order overall. C) This reaction is second order overall. D) This reaction is first order in [OH<sup>-</sup>] concentration. E) The rate law is incorrect as OH<sup>-</sup> is neither a reactant nor a product.](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_12c7_827e_7557476c35e6_TB9687_11.jpg)
A) This reaction is first order overall.
B) This reaction is third order overall.
C) This reaction is second order overall.
D) This reaction is first order in [OH-] concentration.
E) The rate law is incorrect as OH- is neither a reactant nor a product.
![<strong>For the reaction I<sup>-</sup>(aq) + OCl<sup>-</sup>(aq) \rarr IO<sup>-</sup>(aq) + Cl<sup>-</sup>(aq) in basic solution, it was found that </strong> A) This reaction is first order overall. B) This reaction is third order overall. C) This reaction is second order overall. D) This reaction is first order in [OH<sup>-</sup>] concentration. E) The rate law is incorrect as OH<sup>-</sup> is neither a reactant nor a product.](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_12c7_827e_7557476c35e6_TB9687_11.jpg)
A) This reaction is first order overall.
B) This reaction is third order overall.
C) This reaction is second order overall.
D) This reaction is first order in [OH-] concentration.
E) The rate law is incorrect as OH- is neither a reactant nor a product.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
24
A 1.66 x 10-4 mole sample of 239Pu undergoes 9 x 107 decays per second obeying first-order kinetics. How many decays per second would be expected from a 5.46 x 10-1 mole sample?
A) 9 x 108 decays/second
B) 3 x 109 decays/second
C) 9 x 1010 decays/second
D) 3 x 1011 decays/second
E) 9 x 1011 decays/second
A) 9 x 108 decays/second
B) 3 x 109 decays/second
C) 9 x 1010 decays/second
D) 3 x 1011 decays/second
E) 9 x 1011 decays/second

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
25
The reaction A + 2B products was found to have the rate law;
rate = k[A] [B]2. While holding the concentration of A constant, the concentration of B was increased from 0.010M to 0.030M. Predict by what factor the rate of reaction will increase.
A) 3
B) 6
C) 9
D) 30
E) 27
rate = k[A] [B]2. While holding the concentration of A constant, the concentration of B was increased from 0.010M to 0.030M. Predict by what factor the rate of reaction will increase.
A) 3
B) 6
C) 9
D) 30
E) 27

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
26
The first-order rate constant for the decomposition of trioxane (C3H6O3) is known to be 3.05 x 10-4 s-1 at 519ºK. What is the half life of trioxane at 519ºK?
A) 3.28 x 103 s
B) 6.93 x103 s
C) 0.693 hour
D) 0.631 hour
E) 0.328 hour
A) 3.28 x 103 s
B) 6.93 x103 s
C) 0.693 hour
D) 0.631 hour
E) 0.328 hour

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
27
Butadiene reacts to form its dimmer according to the following reaction: ![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60e8_827e_69ce9e45369e_TB9687_00.jpg) Concentration versus time data were collected for this reaction and a plot of 1/[C4H6] resulted in a straight line with slope 6.14 x10-2 M s-1. The integrated for of the rate law is
Concentration versus time data were collected for this reaction and a plot of 1/[C4H6] resulted in a straight line with slope 6.14 x10-2 M s-1. The integrated for of the rate law is
A)![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60e9_827e_39569506fea5_TB9687_11.jpg)
B)![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60ea_827e_13f43cb98b38_TB9687_11.jpg)
C)![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60eb_827e_5fe465261de8_TB9687_11.jpg)
D)![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60ec_827e_673a52fa4d98_TB9687_11.jpg)
E)![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60ed_827e_81ac61cda52b_TB9687_11.jpg)
![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60e8_827e_69ce9e45369e_TB9687_00.jpg) Concentration versus time data were collected for this reaction and a plot of 1/[C4H6] resulted in a straight line with slope 6.14 x10-2 M s-1. The integrated for of the rate law is
Concentration versus time data were collected for this reaction and a plot of 1/[C4H6] resulted in a straight line with slope 6.14 x10-2 M s-1. The integrated for of the rate law isA)
![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60e9_827e_39569506fea5_TB9687_11.jpg)
B)
![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60ea_827e_13f43cb98b38_TB9687_11.jpg)
C)
![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60eb_827e_5fe465261de8_TB9687_11.jpg)
D)
![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60ec_827e_673a52fa4d98_TB9687_11.jpg)
E)
![<strong>Butadiene reacts to form its dimmer according to the following reaction: Concentration versus time data were collected for this reaction and a plot of 1/[C<sub>4</sub>H<sub>6</sub>] resulted in a straight line with slope 6.14 x10<sup>-2</sup> M s<sup>-1</sup>. The integrated for of the rate law is</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_60ed_827e_81ac61cda52b_TB9687_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
28
It is determined that the charcoal in a fire pit used as an ancient hearth has lost about 42.3% of the initial 14C. How old was the fire pit if 14C has a half life of 5730 years?
A) 2210 years
B) 4430 years
C) 4550 years
D) 5250 years
E) 7750 years
A) 2210 years
B) 4430 years
C) 4550 years
D) 5250 years
E) 7750 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
29
The following reaction takes place at 80.1°C:
Ru(NH3)5Cl2+ (aq) + H2O (l) Ru(NH3)5(H2O)3+ (aq) + Cl- (aq)
The following time and concentration data are collected: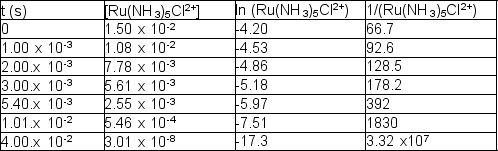 Which of the following is the correct value of the rate constant?
Which of the following is the correct value of the rate constant?
A) 3.28 1/M•s
B) 4.19 1/s
C) 419 1/s
D) 328 1/M•s
E) 328 1/s
Ru(NH3)5Cl2+ (aq) + H2O (l) Ru(NH3)5(H2O)3+ (aq) + Cl- (aq)
The following time and concentration data are collected:
 Which of the following is the correct value of the rate constant?
Which of the following is the correct value of the rate constant?A) 3.28 1/M•s
B) 4.19 1/s
C) 419 1/s
D) 328 1/M•s
E) 328 1/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
30
Nitrous oxide, N2O, decomposes on metal surfaces readily at high temperatures following first-order kinetics for the equation:
2 N2O (g) 2 N2 (g) + O2 (g)
The following data are obtained for a reaction at 850°C: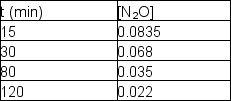 What was the initial concentration of N2O (t = 0)?
What was the initial concentration of N2O (t = 0)?
A) 0.0885 M
B) 0.0940 M
C) 0.101 M
D) 0.112 M
E) 0.123 M
2 N2O (g) 2 N2 (g) + O2 (g)
The following data are obtained for a reaction at 850°C:
 What was the initial concentration of N2O (t = 0)?
What was the initial concentration of N2O (t = 0)?A) 0.0885 M
B) 0.0940 M
C) 0.101 M
D) 0.112 M
E) 0.123 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
31
The following are initial rate data for
2 NO + 2 H2 (\rarr\) N2 + 2 H2O![<strong>The following are initial rate data for 2 NO + 2 H<sub>2</sub> (\rarr\) N<sub>2</sub> + 2 H<sub>2</sub>O What is the rate law?</strong> A) Rate = k[NO] B) Rate = k[H<sub>2</sub>] C) Rate = k[NO][H<sub>2</sub>] D) Rate = k[NO]<sup>2</sup>[H<sub>2</sub>] E) Rate = k[NO][H<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_8800_827e_b9047d6f58fb_TB9687_00.jpg) What is the rate law?
What is the rate law?
A) Rate = k[NO]
B) Rate = k[H2]
C) Rate = k[NO][H2]
D) Rate = k[NO]2[H2]
E) Rate = k[NO][H2]2
2 NO + 2 H2 (\rarr\) N2 + 2 H2O
![<strong>The following are initial rate data for 2 NO + 2 H<sub>2</sub> (\rarr\) N<sub>2</sub> + 2 H<sub>2</sub>O What is the rate law?</strong> A) Rate = k[NO] B) Rate = k[H<sub>2</sub>] C) Rate = k[NO][H<sub>2</sub>] D) Rate = k[NO]<sup>2</sup>[H<sub>2</sub>] E) Rate = k[NO][H<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_8800_827e_b9047d6f58fb_TB9687_00.jpg) What is the rate law?
What is the rate law?A) Rate = k[NO]
B) Rate = k[H2]
C) Rate = k[NO][H2]
D) Rate = k[NO]2[H2]
E) Rate = k[NO][H2]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
32
The reaction of NO with O2 to give oxygen is known to follow a third order rate law(rate = k[NO]2[O2]). Two possible mechanisms are shown below: ![<strong>The reaction of NO with O<sub>2</sub> to give oxygen is known to follow a third order rate law(rate = k[NO]<sup>2</sup>[O<sub>2</sub>]). Two possible mechanisms are shown below: Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?</strong> A) Mechanism 1 because it is simpler B) Mechanism 1 because its rate law is the same as the known rate law C) Mechanism 2 because it only involves 2 steps D) Mechanism 2 because only bimolecular processes are involved E) Mechanism 1 because no unstable species are formed](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_af11_827e_a1cabc6566d2_TB9687_00.jpg) Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?
Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?
A) Mechanism 1 because it is simpler
B) Mechanism 1 because its rate law is the same as the known rate law
C) Mechanism 2 because it only involves 2 steps
D) Mechanism 2 because only bimolecular processes are involved
E) Mechanism 1 because no unstable species are formed
![<strong>The reaction of NO with O<sub>2</sub> to give oxygen is known to follow a third order rate law(rate = k[NO]<sup>2</sup>[O<sub>2</sub>]). Two possible mechanisms are shown below: Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?</strong> A) Mechanism 1 because it is simpler B) Mechanism 1 because its rate law is the same as the known rate law C) Mechanism 2 because it only involves 2 steps D) Mechanism 2 because only bimolecular processes are involved E) Mechanism 1 because no unstable species are formed](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_af11_827e_a1cabc6566d2_TB9687_00.jpg) Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?
Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?A) Mechanism 1 because it is simpler
B) Mechanism 1 because its rate law is the same as the known rate law
C) Mechanism 2 because it only involves 2 steps
D) Mechanism 2 because only bimolecular processes are involved
E) Mechanism 1 because no unstable species are formed

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
33
The following mechanism has been suggested for the decomposition of ozone, O3.O3(g)  O2(g) + O(g) (fast equilibrium)
O2(g) + O(g) (fast equilibrium)
O(g) + O3(g) 2 O2(g) (slow)
Consider the following statements in light of this mechanism:
I. The rate law is second order in O3.
II. The rate does not depend on the concentration of O2.
III. The reaction slows with increased O2 concentration.
IV. The rate law is second order.
V. Substances reacting with O atoms will speed up the reaction.Which of the above statements are true?
A) II and V
B) II only
C) I and IV
D) I and III
E) II, IV and V
 O2(g) + O(g) (fast equilibrium)
O2(g) + O(g) (fast equilibrium)O(g) + O3(g) 2 O2(g) (slow)
Consider the following statements in light of this mechanism:
I. The rate law is second order in O3.
II. The rate does not depend on the concentration of O2.
III. The reaction slows with increased O2 concentration.
IV. The rate law is second order.
V. Substances reacting with O atoms will speed up the reaction.Which of the above statements are true?
A) II and V
B) II only
C) I and IV
D) I and III
E) II, IV and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the rate law associated with the following mechanism:
HCl + HCl![<strong>What is the rate law associated with the following mechanism: HCl + HCl H<sub>2</sub>Cl<sub>2</sub> HCl + CH<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub> H<sub>2</sub>Cl<sub>2</sub> + CH<sub>3</sub>CHClCH<sub>3</sub> \rarr CH<sub>3</sub>CHClCH<sub>3</sub> +2 HCl Net: HCl(g) + CH<sub>3</sub>CHCH<sub>2</sub>(g) \rarr CH<sub>3</sub>CHClCH<sub>3</sub> (g) </strong> A) rate = k[HCl]<sup>2</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] B) rate = k[HCl]<sup>3</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] C) rate = k[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub>] D) rate = k[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] E) rate = k[HCl]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_af13_827e_21c2cf43f50a_TB9687_11.jpg) H2Cl2
H2Cl2
HCl + CH3CHCH2![<strong>What is the rate law associated with the following mechanism: HCl + HCl H<sub>2</sub>Cl<sub>2</sub> HCl + CH<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub> H<sub>2</sub>Cl<sub>2</sub> + CH<sub>3</sub>CHClCH<sub>3</sub> \rarr CH<sub>3</sub>CHClCH<sub>3</sub> +2 HCl Net: HCl(g) + CH<sub>3</sub>CHCH<sub>2</sub>(g) \rarr CH<sub>3</sub>CHClCH<sub>3</sub> (g) </strong> A) rate = k[HCl]<sup>2</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] B) rate = k[HCl]<sup>3</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] C) rate = k[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub>] D) rate = k[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] E) rate = k[HCl]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_af14_827e_5516793e7b35_TB9687_11.jpg) CH3CHClCH3
CH3CHClCH3
H2Cl2 + CH3CHClCH3 CH3CHClCH3 +2 HCl
Net: HCl(g) + CH3CHCH2(g) CH3CHClCH3 (g)
A) rate = k[HCl]2[CH3CHCH2]
B) rate = k[HCl]3[CH3CHCH2]
C) rate = k[H2Cl2][CH3CHClCH3]
D) rate = k[HCl][CH3CHCH2]
E) rate = k[HCl]2
HCl + HCl
![<strong>What is the rate law associated with the following mechanism: HCl + HCl H<sub>2</sub>Cl<sub>2</sub> HCl + CH<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub> H<sub>2</sub>Cl<sub>2</sub> + CH<sub>3</sub>CHClCH<sub>3</sub> \rarr CH<sub>3</sub>CHClCH<sub>3</sub> +2 HCl Net: HCl(g) + CH<sub>3</sub>CHCH<sub>2</sub>(g) \rarr CH<sub>3</sub>CHClCH<sub>3</sub> (g) </strong> A) rate = k[HCl]<sup>2</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] B) rate = k[HCl]<sup>3</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] C) rate = k[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub>] D) rate = k[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] E) rate = k[HCl]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_af13_827e_21c2cf43f50a_TB9687_11.jpg) H2Cl2
H2Cl2HCl + CH3CHCH2
![<strong>What is the rate law associated with the following mechanism: HCl + HCl H<sub>2</sub>Cl<sub>2</sub> HCl + CH<sub>3</sub>CHCH<sub>2</sub> CH<sub>3</sub>CHClCH<sub>3</sub> H<sub>2</sub>Cl<sub>2</sub> + CH<sub>3</sub>CHClCH<sub>3</sub> \rarr CH<sub>3</sub>CHClCH<sub>3</sub> +2 HCl Net: HCl(g) + CH<sub>3</sub>CHCH<sub>2</sub>(g) \rarr CH<sub>3</sub>CHClCH<sub>3</sub> (g) </strong> A) rate = k[HCl]<sup>2</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] B) rate = k[HCl]<sup>3</sup>[CH<sub>3</sub>CHCH<sub>2</sub>] C) rate = k[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub>] D) rate = k[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] E) rate = k[HCl]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d441_af14_827e_5516793e7b35_TB9687_11.jpg) CH3CHClCH3
CH3CHClCH3H2Cl2 + CH3CHClCH3 CH3CHClCH3 +2 HCl
Net: HCl(g) + CH3CHCH2(g) CH3CHClCH3 (g)
A) rate = k[HCl]2[CH3CHCH2]
B) rate = k[HCl]3[CH3CHCH2]
C) rate = k[H2Cl2][CH3CHClCH3]
D) rate = k[HCl][CH3CHCH2]
E) rate = k[HCl]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
35
Hydrogen and iodine react to form HI. One possible mechanism is shown below:I2(g)  2 I(g)
2 I(g)
H2(g) + 2 I(g) 2 HI(g)
Consider the following statements in light of this mechanism:
I. The rate law overall is second order.
II. The iodine atom is an intermediate.
III. The first step is the rate determining step.
IV. The second step is the fast step.
V. The second step is rate determining.
Which of the above statements are true?
A) II, III and IV
B) II and V
C) all except V
D) I and II only
E) I, II and V
 2 I(g)
2 I(g)H2(g) + 2 I(g) 2 HI(g)
Consider the following statements in light of this mechanism:
I. The rate law overall is second order.
II. The iodine atom is an intermediate.
III. The first step is the rate determining step.
IV. The second step is the fast step.
V. The second step is rate determining.
Which of the above statements are true?
A) II, III and IV
B) II and V
C) all except V
D) I and II only
E) I, II and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
36
Heterogeneous catalysts are used in industrial processes because
I. they utilize more of the catalyst atoms.
II. it is easier to separate the products from the catalyst.
III. higher operating temperatures are readily obtained.
IV. they are more selective.Which of the above statements are true?
A) I, II and III
B) II and III
C) III and IV
D) I and IV
E) II and IV
I. they utilize more of the catalyst atoms.
II. it is easier to separate the products from the catalyst.
III. higher operating temperatures are readily obtained.
IV. they are more selective.Which of the above statements are true?
A) I, II and III
B) II and III
C) III and IV
D) I and IV
E) II and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
37
In which order do the following steps typically occur for reactions facilitated by heterogeneous catalysts:
I. Desorption of material
II. Adsorption on materials on catalyst surface
III. Reaction to form products
IV. Movement of bound species over catalyst surface
A) II, I, III, IV
B) II, IV, III, I
C) II, I, IV, III
D) II, III, IV, I
E) IV, II, III, I
I. Desorption of material
II. Adsorption on materials on catalyst surface
III. Reaction to form products
IV. Movement of bound species over catalyst surface
A) II, I, III, IV
B) II, IV, III, I
C) II, I, IV, III
D) II, III, IV, I
E) IV, II, III, I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following does NOT occur during a reaction facilitated by a heterogeneous catalyst?
A) rejuvenation of catalyst via desorption of products
B) migration of bound reactants over catalyst surface
C) bond reorganization at catalyst surface
D) absorption of reactants on catalyst surface
E) weakening of or breaking of bonds and formation of bonds/interactions with catalyst
A) rejuvenation of catalyst via desorption of products
B) migration of bound reactants over catalyst surface
C) bond reorganization at catalyst surface
D) absorption of reactants on catalyst surface
E) weakening of or breaking of bonds and formation of bonds/interactions with catalyst

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
39
The saturation behaviour of enzyme systems (E + S) suggests that
A) reactions in the pocket/active site are not rate limiting.
B) binding to the active site will never be rate limiting.
C) release of product will never be rate limiting.
D) bimolecular reactions cannot happen.
E) different steps in a mechanism can be rate determining based on concentration.
A) reactions in the pocket/active site are not rate limiting.
B) binding to the active site will never be rate limiting.
C) release of product will never be rate limiting.
D) bimolecular reactions cannot happen.
E) different steps in a mechanism can be rate determining based on concentration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
40
Write the overall equation of reaction for the following mechanism and identify the reaction intermediates:
Cl2 2 Cl•
Cl• + CO COCl
COCl + Cl2 COCl2 + Cl•
2 Cl• Cl2
Cl2 2 Cl•
Cl• + CO COCl
COCl + Cl2 COCl2 + Cl•
2 Cl• Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
41
Write the overall equation of reaction for the following mechanism and identify the reaction intermediates:
2 NO2 NO3 + NO
NO3 + CO CO2 + NO2
2 NO2 NO3 + NO
NO3 + CO CO2 + NO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
42
The industrial production of 2-propanol involves the reaction of propene with sulphuric acid and then water. Write the second step of the mechanism if the following is the first step: 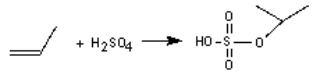
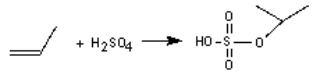

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
43
Draw a molecular picture showing the termolecular process in which two NO molecules collide with an O2 molecule to give two molecules of NO2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
44
Hydrogen peroxide decomposes according to the equation:
2 H2O2(aq) 2 H2O(l) + O2(g)
At 27°C and 1 atm, a 50.0 ml sample of hydrogen peroxide decomposes at a rate that produces 10.0 ml/sec of O2(g). Assuming ideal behavioura) Determine the moles of oxygen produced per second.b) Determine the change in molarity of H2O2 per second.
2 H2O2(aq) 2 H2O(l) + O2(g)
At 27°C and 1 atm, a 50.0 ml sample of hydrogen peroxide decomposes at a rate that produces 10.0 ml/sec of O2(g). Assuming ideal behavioura) Determine the moles of oxygen produced per second.b) Determine the change in molarity of H2O2 per second.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
45
The following concentration vs. time data were collected for the reaction:
A + 2B C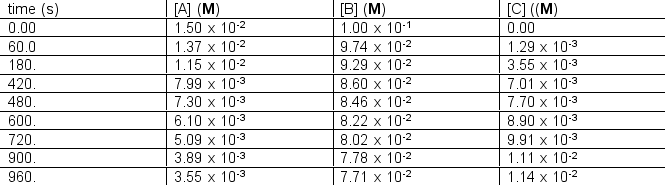 Calculate
Calculate 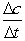 for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
A + 2B C
 Calculate
Calculate 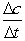 for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
46
Consider the following three molecular pictures that represent the relative numbers of the two reactants involved in one step of the depletion of stratospheric ozone by chlorine atoms: 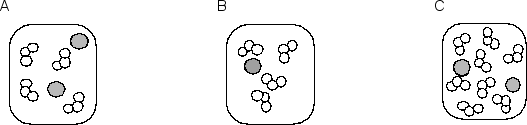 The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2
The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2  If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
 The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2
The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2  If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A? 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
47
The reaction of NO2 with CO to give CO2 and NO can proceed through different mechanisms. What first step would be consistent with the following rate law?Rate = k[NO2][CO]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
48
What are the units of a rate constant for a reaction that has an overall order of 3?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
49
The reaction of NO2 with CO to give CO2 and NO can proceed through different mechanisms. What rate law would be consistent for the following first step?
2 NO2 NO + NO3
2 NO2 NO + NO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
50
The rate law for the reaction of NO with O2 to give NO2 is shown below:rate = k [NO]2[O2]
a) If all other conditions are kept constant, what will be the effect on the rate if the concentration of NO is doubled?
b) If all other conditions are kept constant, what will be the effect on the rate if the concentration of O2 is doubled?
a) If all other conditions are kept constant, what will be the effect on the rate if the concentration of NO is doubled?
b) If all other conditions are kept constant, what will be the effect on the rate if the concentration of O2 is doubled?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
51
At moderate temperatures, the rate law for the reaction of NO2 and CO to give CO2 and NO follows the rate law shown below:rate = k [NO2]2 ![At moderate temperatures, the rate law for the reaction of NO<sub>2</sub> and CO to give CO<sub>2</sub> and NO follows the rate law shown below:rate = k [NO<sub>2</sub>]<sup>2</sup> In which flask will the reaction be faster and how much faster?](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d443_0eae_827e_b9db2beea02d_TB9687_00.jpg) In which flask will the reaction be faster and how much faster?
In which flask will the reaction be faster and how much faster?
![At moderate temperatures, the rate law for the reaction of NO<sub>2</sub> and CO to give CO<sub>2</sub> and NO follows the rate law shown below:rate = k [NO<sub>2</sub>]<sup>2</sup> In which flask will the reaction be faster and how much faster?](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d443_0eae_827e_b9db2beea02d_TB9687_00.jpg) In which flask will the reaction be faster and how much faster?
In which flask will the reaction be faster and how much faster?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
52
Radioactive decay follows first-order kinetics. Some smoke detectors use the isotope 241Am that has a half-life of 432.2 years. In how many years will 95% of the 241Am have decayed?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
53
Trioxane undergoes decomposition to formaldehyde at elevated temperatures.C3H6O3 (g) 3 CH2O (g)The following data was collected for the gas phase reaction at 519ºK: 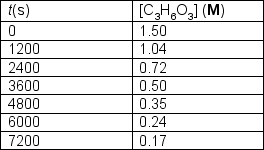 Determine the order of the decomposition in trioxane and the rate constant at 519ºK.
Determine the order of the decomposition in trioxane and the rate constant at 519ºK.
 Determine the order of the decomposition in trioxane and the rate constant at 519ºK.
Determine the order of the decomposition in trioxane and the rate constant at 519ºK. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
54
Ammonium cyanate undergoes rearrangement to form urea in aqueous solution.NH4NCO(aq) CO(NH2)2 (aq)The following data was collected: 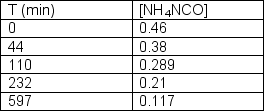 Determine the order of the reaction and the rate constant.
Determine the order of the reaction and the rate constant.
 Determine the order of the reaction and the rate constant.
Determine the order of the reaction and the rate constant. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
55
Sucrose, cane sugar, reacts with water in acid solution to give glucose and fructose, which have the same chemical formula.
C12H22O11 (aq) + H2O (l) 2 C6H12O6 (aq)
The following data were obtained at room temperature for sucrose: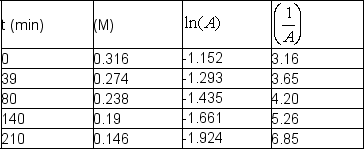 Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
C12H22O11 (aq) + H2O (l) 2 C6H12O6 (aq)
The following data were obtained at room temperature for sucrose:
 Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
56
Rate data were collected for the following reaction at a constant temperature.2ClO2(aq) + 2 OH-1(aq) ClO3-1(aq) + ClO2-1(aq) + H2O(l) 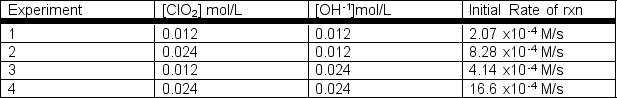 a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
 a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
57
Nitrous oxide, N2O, decomposes on metal surfaces readily at high temperatures following first-order kinetics for the equation:
2 N2O (g) 2 N2 (g) + O2 (g)
The following data are obtained for a reaction at 850°C: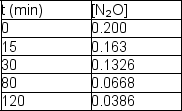 Determine the rate constant and half-life for the reaction.
Determine the rate constant and half-life for the reaction.
2 N2O (g) 2 N2 (g) + O2 (g)
The following data are obtained for a reaction at 850°C:
 Determine the rate constant and half-life for the reaction.
Determine the rate constant and half-life for the reaction. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
58
The following are initial rate data for 2 NO + 2 H2 N2 + 2 H2O ![The following are initial rate data for 2 NO + 2 H<sub>2</sub> \rightarrow N<sub>2</sub> + 2 H<sub>2</sub>O The rate law is determined to be: rate = k[NO]<sup>2</sup>[H<sub>2</sub>]. With this information determine k using the data from experiment 2.](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d443_5bd4_827e_b997e40295b0_TB9687_00.jpg) The rate law is determined to be: rate = k[NO]2[H2]. With this information determine k using the data from experiment 2.
The rate law is determined to be: rate = k[NO]2[H2]. With this information determine k using the data from experiment 2.
![The following are initial rate data for 2 NO + 2 H<sub>2</sub> \rightarrow N<sub>2</sub> + 2 H<sub>2</sub>O The rate law is determined to be: rate = k[NO]<sup>2</sup>[H<sub>2</sub>]. With this information determine k using the data from experiment 2.](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d443_5bd4_827e_b997e40295b0_TB9687_00.jpg) The rate law is determined to be: rate = k[NO]2[H2]. With this information determine k using the data from experiment 2.
The rate law is determined to be: rate = k[NO]2[H2]. With this information determine k using the data from experiment 2. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
59
Assume that the following first-order reaction has a rate constant k = 0.0137/min:
SO2Cl2 SO2 + Cl2
Given the initial [SO2Cl2] = 0.42 M, how many minutes will it take for [SO2Cl2] = 0.19 M?
SO2Cl2 SO2 + Cl2
Given the initial [SO2Cl2] = 0.42 M, how many minutes will it take for [SO2Cl2] = 0.19 M?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
60
The three reactions below, with identical reaction stoichiometry, must all share the same third order rate law found for the reaction of NO and O2. True or False, and why?
2 NO(g) + O2(g) 2 NO2(g)2
NO(g) + Cl2(g) 2 NOCl(g)2
NO(g) + F2(g) 2 NOF(g)
2 NO(g) + O2(g) 2 NO2(g)2
NO(g) + Cl2(g) 2 NOCl(g)2
NO(g) + F2(g) 2 NOF(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
61
Nitrogen dioxide, NO2 will react with carbon monoxide, CO, to form nitric oxide, NO, and carbon dioxide, CO2. A proposed mechanism is:
2NO2 NO3 + NO
NO3 + CO NO2 + CO2
Experiments indicate that the rate of the reaction is independent of the CO concentration. Identify the rate determining step and derive the rate law consistent with the mechanism and experimental observation.
2NO2 NO3 + NO
NO3 + CO NO2 + CO2
Experiments indicate that the rate of the reaction is independent of the CO concentration. Identify the rate determining step and derive the rate law consistent with the mechanism and experimental observation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
62
A proposed mechanism for the following reaction, A2 + B2 2AB, isA2  2AA + B2 AB + B slowB +A2 AB + ADetermine the rate law.
2AA + B2 AB + B slowB +A2 AB + ADetermine the rate law.
 2AA + B2 AB + B slowB +A2 AB + ADetermine the rate law.
2AA + B2 AB + B slowB +A2 AB + ADetermine the rate law. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
63
The reaction of nitrogen dioxide and fluorine is:2 NO2 + F2 2 NO2FOne proposed mechanism has two steps; the first step is rate determining: 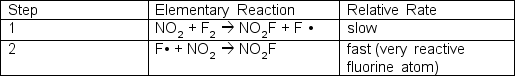 What is the experimentally determined rate law?
What is the experimentally determined rate law?
 What is the experimentally determined rate law?
What is the experimentally determined rate law? 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
64
The rate law for the reaction2 H2 (g) + 2 NO (g) N2 (g) + 2 H2O (g)is rate = k[H2 ][NO]2. Which of the following mechanisms can be ruled out because the derived rate law is NOT consistent with the observed rate law?Mechanism 1H2 + NO N + H2O (slow)N + NO N2 + O (fast)O + H2 H2O (fast)Mechanism 2H2 + 2 NO N2O + H2O (slow)N2O + H2 N2 + H2O (fast)Mechanism 32 NO ![The rate law for the reaction2 H<sub>2</sub> (g) + 2 NO (g) \rightarrow N<sub>2</sub> (g) + 2 H<sub>2</sub>O (g)is rate = k[H<sub>2</sub> ][NO]<sup>2</sup>. Which of the following mechanisms can be ruled out because the derived rate law is NOT consistent with the observed rate law?Mechanism 1H<sub>2</sub> + NO \rightarrow N + H<sub>2</sub>O (slow)N + NO \rightarrow N<sub>2</sub> + O (fast)O + H<sub>2</sub> \rightarrow H<sub>2</sub>O (fast)Mechanism 2H<sub>2</sub> + 2 NO \rightarrow N<sub>2</sub>O + H<sub>2</sub>O (slow)N<sub>2</sub>O + H<sub>2</sub> \rightarrow N<sub>2</sub> + H<sub>2</sub>O (fast)Mechanism 32 NO N<sub>2</sub>O<sub>2</sub> (fast equilibrium)N<sub>2</sub>O<sub>2</sub><sub> </sub>+ H<sub>2</sub> \rightarrow N<sub>2</sub>O + H<sub>2</sub>O (slow)N<sub>2</sub>O + H<sub>2</sub> \rightarrow N<sub>2</sub> + H<sub>2</sub>O (fast)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d443_82e7_827e_b71252684bbc_TB9687_11.jpg) N2O2 (fast equilibrium)N2O2 + H2 N2O + H2O (slow)N2O + H2 N2 + H2O (fast)
N2O2 (fast equilibrium)N2O2 + H2 N2O + H2O (slow)N2O + H2 N2 + H2O (fast)
![The rate law for the reaction2 H<sub>2</sub> (g) + 2 NO (g) \rightarrow N<sub>2</sub> (g) + 2 H<sub>2</sub>O (g)is rate = k[H<sub>2</sub> ][NO]<sup>2</sup>. Which of the following mechanisms can be ruled out because the derived rate law is NOT consistent with the observed rate law?Mechanism 1H<sub>2</sub> + NO \rightarrow N + H<sub>2</sub>O (slow)N + NO \rightarrow N<sub>2</sub> + O (fast)O + H<sub>2</sub> \rightarrow H<sub>2</sub>O (fast)Mechanism 2H<sub>2</sub> + 2 NO \rightarrow N<sub>2</sub>O + H<sub>2</sub>O (slow)N<sub>2</sub>O + H<sub>2</sub> \rightarrow N<sub>2</sub> + H<sub>2</sub>O (fast)Mechanism 32 NO N<sub>2</sub>O<sub>2</sub> (fast equilibrium)N<sub>2</sub>O<sub>2</sub><sub> </sub>+ H<sub>2</sub> \rightarrow N<sub>2</sub>O + H<sub>2</sub>O (slow)N<sub>2</sub>O + H<sub>2</sub> \rightarrow N<sub>2</sub> + H<sub>2</sub>O (fast)](https://d2lvgg3v3hfg70.cloudfront.net/TB9687/11ee726d_d443_82e7_827e_b71252684bbc_TB9687_11.jpg) N2O2 (fast equilibrium)N2O2 + H2 N2O + H2O (slow)N2O + H2 N2 + H2O (fast)
N2O2 (fast equilibrium)N2O2 + H2 N2O + H2O (slow)N2O + H2 N2 + H2O (fast) 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
65
For the following reaction A + B C + D, the rate law is determined to be Rate = k[A]2a) Of the five proposed mechanisms shown below, which is consistent with the experimentally determined rate law?
1. 2 A Z (slow)2 B + Z 2 C + 2 D (fast)
2. A + B C + D (slow)
3. 2 B N (slow)2 A + N 2 C + 2 D (fast)
4. A X (slow)B + X C + D (fast)
5. B M (slow)A + M C + D (fast)b)
Are there any intermediates in the mechanism you chose and if so what?
1. 2 A Z (slow)2 B + Z 2 C + 2 D (fast)
2. A + B C + D (slow)
3. 2 B N (slow)2 A + N 2 C + 2 D (fast)
4. A X (slow)B + X C + D (fast)
5. B M (slow)A + M C + D (fast)b)
Are there any intermediates in the mechanism you chose and if so what?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
66
In the formation of dinitrogentetroxide, two NO2 molecules react to make an N-N bond.2 NO2 2 N2O4Draw molecular pictures superimposed on a diagram of energy vs. reaction coordinate that illustrates this process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
67
The following rate constants were obtained at the stated temperatures for the first-order reaction:A B  Find the activation energy (in kJ/mole) for this reaction.
Find the activation energy (in kJ/mole) for this reaction.
 Find the activation energy (in kJ/mole) for this reaction.
Find the activation energy (in kJ/mole) for this reaction. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
68
Nitrogen dioxide molecules undergo oxygen exchange with an activation energy of 100 kJ/mole. By how much will the reaction rate constant increase if temperature is increased from 25oC to 75oC?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
69
A particular first-order reaction is characterized by activation energy of 50 kJ/mole. At what temperature would the rate of the reaction be 10 times that at 298oK?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
70
The reaction of ozone with oxygen atoms to give oxygen has an activation energy of 17.1 kJ/mole with a rate constant at 298oK, k = 4.8 x 106 1/M•s. Calculate the rate constant for this reaction at 315oK.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
71
The reaction of ozone with oxygen atoms to produce molecular oxygen has a rate constant of 4.8x106 1/M s at 25oC. A 20 degree increase in temperature results in a rate constant of 7.4x106 1/M s. What is the rate constant at 100oC?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
72
The activation energy for the high temperature conversion cyclopropane to propene is 270 kJ mol-1. At what temperature would the rate constant for this reaction be ten times that of 500oC?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
73
The rate constant of the reaction, O (g) + N2 (g) NO (g) + N (g), is 9.7 x 10101/M•s at 800oK and has an activation energy of 315 kJ/mole. What is the value of the rate constant at 700oK?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
74
For a large number of reactions in organic chemistry, an increase in temperature 10°C over room temperature will double the rate. What activation energy does this correspond to?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
75
For a hypothetical reaction, the activation energy is Eact = 50.2 kJ/mol and it has an Arrhenius constant of 22.3 M-1s-1. Determine what the rate constant would be if the temperature was 400 C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
76
Consider the following energy-reaction coordinate diagram. 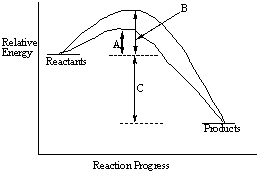 Give the names for the quantities indicated by A, B and C.
Give the names for the quantities indicated by A, B and C.
 Give the names for the quantities indicated by A, B and C.
Give the names for the quantities indicated by A, B and C.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck
77
Write the overall equation of reaction for the following mechanism and identify any reaction intermediates and any catalysts.
H2O2 + I-1 H2O + OI-1
H2O2 + OI-1 H2O + O2 + I-1
H2O2 + I-1 H2O + OI-1
H2O2 + OI-1 H2O + O2 + I-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 77 في هذه المجموعة.
فتح الحزمة
k this deck








