Deck 4: Atoms and Light
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/81
العب
ملء الشاشة (f)
Deck 4: Atoms and Light
1
Understand some of the fundamental aspects of atoms.
Atoms have mass, contain positive nuclei, contain electrons, and occupy volume.
2
Understand some of the fundamental aspects of light.
Light has wave-like and particle-like characteristics.
3
Explain the origins of atomic spectra and relate electron energies in the hydrogen atom to its emission spectrum.
The energies of electrons in atoms are quantized.
4
Describe properties of free electrons and those in atoms or molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
5
Write valid sets of quantum numbers for a given set of orbitals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
6
Recognize shapes of s, p, d, and f orbitals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
7
Describe the major chemical reactions in the troposphere and stratosphere and explain the chemistry of the greenhouse effect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
8
One of the characteristics of atoms is that they attract one another. What characteristic of gases supports this notion?
A) Equal numbers of molecules occupy equal volumes.
B) Gas molecules have a small, but definite volume.
C) The volume of a gas increases with increasing temperature.
D) Non-ideal behaviour is observed at low temperatures.
E) Volume decreases with increasing pressure.
A) Equal numbers of molecules occupy equal volumes.
B) Gas molecules have a small, but definite volume.
C) The volume of a gas increases with increasing temperature.
D) Non-ideal behaviour is observed at low temperatures.
E) Volume decreases with increasing pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
9
The volume of an atom is determined by
A) the number of protons in the nucleus.
B) the number of protons and neutrons in the nucleus.
C) the number of electrons in the nucleus.
D) the electron cloud surrounding the nucleus.
E) the period it occupies in the periodic table.
A) the number of protons in the nucleus.
B) the number of protons and neutrons in the nucleus.
C) the number of electrons in the nucleus.
D) the electron cloud surrounding the nucleus.
E) the period it occupies in the periodic table.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
10
The mass of an atom is determined by
A) the number of protons in the nucleus.
B) the number of protons and neutrons in the nucleus.
C) the number of electrons in the electron cloud surrounding the nucleus.
D) the number of neutrons and electrons.
E) the period it occupies in the periodic table.
A) the number of protons in the nucleus.
B) the number of protons and neutrons in the nucleus.
C) the number of electrons in the electron cloud surrounding the nucleus.
D) the number of neutrons and electrons.
E) the period it occupies in the periodic table.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
11
Most of the volume of an atom is made up of
A) electrons.
B) protons.
C) neutrons.
D) protons and neutrons.
E) empty space.
A) electrons.
B) protons.
C) neutrons.
D) protons and neutrons.
E) empty space.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
12
When a gas such as argon condenses
A) atoms combine with one another to form chemical bonds.
B) atoms are attracted to one another due to electrical forces acting between electrons on one atom and protons of the other.
C) electrons of one atom repel those on another causing the gas to condense and form a liquid.
D) atoms are attracted to one another due to electrical forces acting between electrons on one atom and neutrons of the other.
E) atoms are attracted to one another due to electrical forces acting between neutrons on each of the atoms.
A) atoms combine with one another to form chemical bonds.
B) atoms are attracted to one another due to electrical forces acting between electrons on one atom and protons of the other.
C) electrons of one atom repel those on another causing the gas to condense and form a liquid.
D) atoms are attracted to one another due to electrical forces acting between electrons on one atom and neutrons of the other.
E) atoms are attracted to one another due to electrical forces acting between neutrons on each of the atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
13
Use the following equations for Questions
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Which of the following provides the greatest energy?
A) 5.00 x 1010 photons of frequency 1.00 x109 1/s
B) 7.00 x 106 photons of wavelength 550 nm
C) 5.00 x 105 photons of frequency 1.05 x 1014 1/s
D) 7.20 x108 photons of wavelength 200 nm
E) 5.00 x 1015 photons of wavelength 15 m
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Which of the following provides the greatest energy?
A) 5.00 x 1010 photons of frequency 1.00 x109 1/s
B) 7.00 x 106 photons of wavelength 550 nm
C) 5.00 x 105 photons of frequency 1.05 x 1014 1/s
D) 7.20 x108 photons of wavelength 200 nm
E) 5.00 x 1015 photons of wavelength 15 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
14
Use the following equations for Questions
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Which of the following radiation is the most energetic?
A) radiation of wavelength 500 nm
B) radiation of frequency 5.76 x1014 Hz
C) radiation of frequency 8.25 x1014 Hz
D) radiation of wavelength 250 nm
E) cannot be determined based on the information given
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Which of the following radiation is the most energetic?
A) radiation of wavelength 500 nm
B) radiation of frequency 5.76 x1014 Hz
C) radiation of frequency 8.25 x1014 Hz
D) radiation of wavelength 250 nm
E) cannot be determined based on the information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
15
Use the following equations for Questions
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Sodium emits yellow light of wavelength 690nm when heated in a flame. What is the frequency of this light?
A) 2.07 x 1011 s-1
B) 4.35 x 105 s-1
C) 4.35 x1014 s-1
D) 207 nm
E) 1.04 x 1014 s-1
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Sodium emits yellow light of wavelength 690nm when heated in a flame. What is the frequency of this light?
A) 2.07 x 1011 s-1
B) 4.35 x 105 s-1
C) 4.35 x1014 s-1
D) 207 nm
E) 1.04 x 1014 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
16
Use the following equations for Questions
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Chromium metal has a binding energy of 7.21 x 10-19J for certain electrons. What is the photon frequency needed to eject electrons with 2.2 x 10-19J of energy?
A) 1.4 x 1015 s-1
B) 2.4 x 1015 s-1
C) 5.5 x 1015 s-1
D) 1.2 x 1014 s-1
E) 1.3 x 1016 s-1
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Chromium metal has a binding energy of 7.21 x 10-19J for certain electrons. What is the photon frequency needed to eject electrons with 2.2 x 10-19J of energy?
A) 1.4 x 1015 s-1
B) 2.4 x 1015 s-1
C) 5.5 x 1015 s-1
D) 1.2 x 1014 s-1
E) 1.3 x 1016 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
17
Use the following equations for Questions
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Chromium metal, binding energy of 7.21 x 10-19J for certain electrons, is subjected to a photon source of frequency 1.4 x 1015 s-1. If the intensity of the photon source in increased, one would expect
A) the energy of the ejected electrons to increase.
B) the energy of the ejected electrons to decrease.
C) the number of ejected electrons to increase.
D) the number of ejected electrons to decrease.
E) no change.
c = 3.0 x108m/s = ;E = h ; h = 6.626 x 10-34 J.s
E = h - h 0; E = ½ mv2 (KE of an electron) masse = 9.11 x 10-31 kg
-Chromium metal, binding energy of 7.21 x 10-19J for certain electrons, is subjected to a photon source of frequency 1.4 x 1015 s-1. If the intensity of the photon source in increased, one would expect
A) the energy of the ejected electrons to increase.
B) the energy of the ejected electrons to decrease.
C) the number of ejected electrons to increase.
D) the number of ejected electrons to decrease.
E) no change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
18
Use the following equations for Questions
;
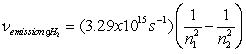 E =
E =
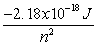 n
n
-If a hydrogen atom is excited into the n = 4 state, how many transitions are possible by emitting electromagnetic radiation?
A) 1
B) 2
C) 3
D) 4
E) 5
;
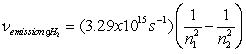 E =
E =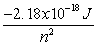 n
n-If a hydrogen atom is excited into the n = 4 state, how many transitions are possible by emitting electromagnetic radiation?
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
19
Use the following equations for Questions
;
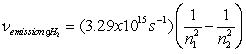 E =
E =
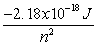 n
n
-An electron in an H atom is excited to an energy level of -8.72 x 10-20 J. What energy level does this correspond with?
A) 2
B) 3
C) 4
D) 5
E) 6
;
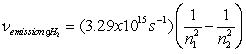 E =
E =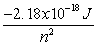 n
n-An electron in an H atom is excited to an energy level of -8.72 x 10-20 J. What energy level does this correspond with?
A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
20
Use the following equations for Questions
;
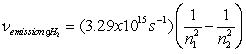 E =
E =
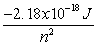 n
n
-An excited electron on a hydrogen atom releases a photon as it falls from energy level 6 to level 3. What is the wavelength of the photon released?
A) 2.74 x1016 s-1
B) 2.18 x10-16 J
C) 1090 nm
D) 109 nm
E) 3 m
;
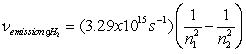 E =
E =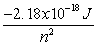 n
n-An excited electron on a hydrogen atom releases a photon as it falls from energy level 6 to level 3. What is the wavelength of the photon released?
A) 2.74 x1016 s-1
B) 2.18 x10-16 J
C) 1090 nm
D) 109 nm
E) 3 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
21
Use the following equations for Questions
;
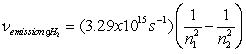 E =
E =
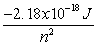 n
n
-An excited electron on a hydrogen atom releases a photon as it falls from energy level 6 to level 3. What is the change in energy of the atom and the energy of the photon released?
A) 1.1 x105 J, 1.1x105 J
B) -1.1x105 J, 1.1x105 J
C) 1.8x10-19 J, 1.8x10-19 J
D) -1.8x10-19 J, -1.8x10-19 J
E) -1.8x10-19 J, 1.8x10-19 J
;
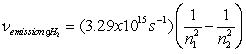 E =
E =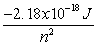 n
n-An excited electron on a hydrogen atom releases a photon as it falls from energy level 6 to level 3. What is the change in energy of the atom and the energy of the photon released?
A) 1.1 x105 J, 1.1x105 J
B) -1.1x105 J, 1.1x105 J
C) 1.8x10-19 J, 1.8x10-19 J
D) -1.8x10-19 J, -1.8x10-19 J
E) -1.8x10-19 J, 1.8x10-19 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
22
Use the following equations for Questions
;
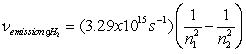 E =
E =
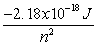 n
n
-A hydrogen atom absorbs a photon resulting in an electron transitioning from the n = 2 to n = 5 energy level. What is the wavelength of the absorbed photon and what is the change in energy of the atom?
A) 434 m, 4.6x10-19 J
B) 434 m, -4.6x10-19 J
C) 434 nm, -4.6x10-19 J
D) 434 nm, 4.6x10-19 J
E) 6.9x1014 s-1, 4.6x10-19 J
;
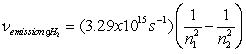 E =
E =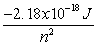 n
n-A hydrogen atom absorbs a photon resulting in an electron transitioning from the n = 2 to n = 5 energy level. What is the wavelength of the absorbed photon and what is the change in energy of the atom?
A) 434 m, 4.6x10-19 J
B) 434 m, -4.6x10-19 J
C) 434 nm, -4.6x10-19 J
D) 434 nm, 4.6x10-19 J
E) 6.9x1014 s-1, 4.6x10-19 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
23
Use the following equations for Questions
Ekinetic = ½ mv2; masse = 9.11 x 10-31kg;
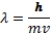
-What is the wavelength of a proton (m = 1.67 x 10-27 kg) moving at 1.2 x 105 m/s?
A) 0.033 nm
B) 3.3 x 10-2 nm
C) 3.3 x 10-9 m
D) 3.3 x 10-12 m
E) 3.3 nm
Ekinetic = ½ mv2; masse = 9.11 x 10-31kg;
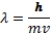
-What is the wavelength of a proton (m = 1.67 x 10-27 kg) moving at 1.2 x 105 m/s?
A) 0.033 nm
B) 3.3 x 10-2 nm
C) 3.3 x 10-9 m
D) 3.3 x 10-12 m
E) 3.3 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
24
Use the following equations for Questions
Ekinetic = ½ mv2; masse = 9.11 x 10-31kg;
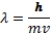
-The Heisenberg uncertainty principle
A) places limits on the accuracy of measuring both position and motion.
B) is most important for microscopic objects.
C) makes the idea of "orbits" for electrons meaningless.
D) a, b
E) a, b and c
Ekinetic = ½ mv2; masse = 9.11 x 10-31kg;
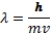
-The Heisenberg uncertainty principle
A) places limits on the accuracy of measuring both position and motion.
B) is most important for microscopic objects.
C) makes the idea of "orbits" for electrons meaningless.
D) a, b
E) a, b and c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
25
Use the following equations for Questions
Ekinetic = ½ mv2; masse = 9.11 x 10-31kg;
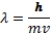
-Which of the following objects would we expect NOT to have any meaningful wave properties?
A) electrons
B) protons
C) neutrons
D) a mole of carbon atoms
E) photons
Ekinetic = ½ mv2; masse = 9.11 x 10-31kg;
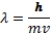
-Which of the following objects would we expect NOT to have any meaningful wave properties?
A) electrons
B) protons
C) neutrons
D) a mole of carbon atoms
E) photons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
26
Use the following equations for Questions
En =

-Which of the following set of quantum numbers is NOT possible?
A) n =2, l = 1, ml = 0, ms =1/2
B) n =3, l = 2, ml = 2, ms =1/2
C) n =4, l = 2, ml = 2, ms = -1/2
D) n =3, l = 3, ml = 2, ms = -1/2
E) n = 2, l = 1, ml = 1, ms = -1/2
En =

-Which of the following set of quantum numbers is NOT possible?
A) n =2, l = 1, ml = 0, ms =1/2
B) n =3, l = 2, ml = 2, ms =1/2
C) n =4, l = 2, ml = 2, ms = -1/2
D) n =3, l = 3, ml = 2, ms = -1/2
E) n = 2, l = 1, ml = 1, ms = -1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
27
Use the following equations for Questions
En =

-Which of the following set of quantum numbers is possible?
A) n =2, l = 0, ml = 1, ms =1/2
B) n =3, l = 3, ml = 2, ms =1/2
C) n =4, l = 3, ml = 3, ms = -1/2
D) n =3, l = 3, ml = 2, ms = -1/2
E) n = 2, l = 1, ml = 2, ms = -1/2
En =

-Which of the following set of quantum numbers is possible?
A) n =2, l = 0, ml = 1, ms =1/2
B) n =3, l = 3, ml = 2, ms =1/2
C) n =4, l = 3, ml = 3, ms = -1/2
D) n =3, l = 3, ml = 2, ms = -1/2
E) n = 2, l = 1, ml = 2, ms = -1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
28
Use the following equations for Questions
En =

-Which of the following sets of quantum numbers is valid for an electron in a 4d orbital?
A) n =4, l=1, ml=1, ms =1/2
B) n =4, l=2, ml=3, ms = -1/2
C) n =4, l=3, ml=2, ms =1/2
D) n =4, l=2, ml=1, ms = -1/2
E) n =4, l=0, ml=0, ms = -1/2
En =

-Which of the following sets of quantum numbers is valid for an electron in a 4d orbital?
A) n =4, l=1, ml=1, ms =1/2
B) n =4, l=2, ml=3, ms = -1/2
C) n =4, l=3, ml=2, ms =1/2
D) n =4, l=2, ml=1, ms = -1/2
E) n =4, l=0, ml=0, ms = -1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
29
Use the following equations for Questions
En =

-Which of the following sets of quantum numbers is valid for an electron in a 4f orbital?
A) n =4, l=1, ml=1, ms =1/2
B) n =4, l=3, ml=4, ms = -1/2
C) n =4, l=3, ml=2, ms =1/2
D) n =4, l=2, ml=1, ms = -1/2
E) n =4, l=4, ml=1, ms = 1/2
En =

-Which of the following sets of quantum numbers is valid for an electron in a 4f orbital?
A) n =4, l=1, ml=1, ms =1/2
B) n =4, l=3, ml=4, ms = -1/2
C) n =4, l=3, ml=2, ms =1/2
D) n =4, l=2, ml=1, ms = -1/2
E) n =4, l=4, ml=1, ms = 1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
30
Use the following equations for Questions
En =

-An electron in an H atom is excited to an energy of -8.72 x 10-20 J which corresponds to a primary level 5. What is the largest value of ml possible for this energy level?
A) 3
B) 4
C) 5
D) 6
E) 9
En =

-An electron in an H atom is excited to an energy of -8.72 x 10-20 J which corresponds to a primary level 5. What is the largest value of ml possible for this energy level?
A) 3
B) 4
C) 5
D) 6
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
31
Use the following equations for Questions
En =

-An electron in an H atom is excited to an energy of -8.72 x 10-20 J which corresponds to a primary level 5. How many possible ml values are there for this energy level?
A) 4
B) 18
C) 5
D) 11
E) 9
En =

-An electron in an H atom is excited to an energy of -8.72 x 10-20 J which corresponds to a primary level 5. How many possible ml values are there for this energy level?
A) 4
B) 18
C) 5
D) 11
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
32
Use the following equations for Questions
En =

-An electron in an H atom is excited to an energy of -2.42 x 10-19 J. What is the largest value of ml possible for this energy level?
A) 1
B) 2
C) 3
D) 0
E) 7
En =

-An electron in an H atom is excited to an energy of -2.42 x 10-19 J. What is the largest value of ml possible for this energy level?
A) 1
B) 2
C) 3
D) 0
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
33
Use the following equations for Questions
En =

-The magnetic quantum number
A) is always less than the value of l.
B) is always less than n.
C) is always greater than ms.
D) correlates strongly with the energy of the electron.
E) must be a positive number.
En =

-The magnetic quantum number
A) is always less than the value of l.
B) is always less than n.
C) is always greater than ms.
D) correlates strongly with the energy of the electron.
E) must be a positive number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
34
Use the following equations for Questions
En =

-The principle quantum number for the outermost 2 electrons in Sr would be
A) 3.
B) 4.
C) 5.
D) 6.
E) 2.
En =

-The principle quantum number for the outermost 2 electrons in Sr would be
A) 3.
B) 4.
C) 5.
D) 6.
E) 2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
35
Use the following equations for Questions
En =

-The principle quantum number for the 3 outermost electrons in Sc are
A) 3, 3 and 3.
B) 4, 4 and 3.
C) 4, 4 and 4.
D) 3, 3, and 4.
E) 4, 4, and 5.
En =

-The principle quantum number for the 3 outermost electrons in Sc are
A) 3, 3 and 3.
B) 4, 4 and 3.
C) 4, 4 and 4.
D) 3, 3, and 4.
E) 4, 4, and 5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
36
Use the following equations for Questions
En =

-The azimuthal quantum number for the outermost electron on K is
A) 0.
B) 1.
C) 2.
D) 3.
E) 4.
En =

-The azimuthal quantum number for the outermost electron on K is
A) 0.
B) 1.
C) 2.
D) 3.
E) 4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many nodes are there in a 3p orbital?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
38
An electron in which subshell will, on average, be closer to the nucleus of an atom?
A) 3s
B) 3p
C) 3d
D) 4d
E) 4s
A) 3s
B) 3p
C) 3d
D) 4d
E) 4s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which atom has a smaller 3s orbital?
A) an atom with more protons
B) an atom with fewer protons
C) an atom with more neutrons
D) an atom with fewer neutrons
E) the size of the 3s orbital is the same for all atoms.
A) an atom with more protons
B) an atom with fewer protons
C) an atom with more neutrons
D) an atom with fewer neutrons
E) the size of the 3s orbital is the same for all atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
40
How many orbitals are there in the d sublevel?
A) 1
B) 3
C) 5
D) 6
E) 7
A) 1
B) 3
C) 5
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
41
The quantum numbers that correspond to size and orientation of orbitals are
A) principal and azimuthal.
B) principal and magnetic.
C) azimuthal and magnetic.
D) n and l.
E) azimuthal and spin.
A) principal and azimuthal.
B) principal and magnetic.
C) azimuthal and magnetic.
D) n and l.
E) azimuthal and spin.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
42
What wavelength photons are required to "split" molecular O2 into O atoms?
A) microwave region of wavelengths
B) infrared region of wavelengths
C) 280-320 nm
D) 240-280 nm
E) 180-220 nm
A) microwave region of wavelengths
B) infrared region of wavelengths
C) 280-320 nm
D) 240-280 nm
E) 180-220 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
43
What is the main reason for CO2's significant participation in global warming?
A) its high concentration
B) its rapid destruction in the atmosphere
C) its efficiency in absorbing visible light
D) its slow uptake into the biosphere
E) its efficiency in absorbing infrared light
A) its high concentration
B) its rapid destruction in the atmosphere
C) its efficiency in absorbing visible light
D) its slow uptake into the biosphere
E) its efficiency in absorbing infrared light

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following reactions is NOT predominant in the thermosphere?
A) N2 +h N2+
B) O2 + h 2 O
C) CO + 1/2 O (\rarr\) CO2
D) 2 O O2 + heat
E) N2+ N2 + heat
A) N2 +h N2+
B) O2 + h 2 O
C) CO + 1/2 O (\rarr\) CO2
D) 2 O O2 + heat
E) N2+ N2 + heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
45
The temperature at the last ice age was how much different than today's average temperature?
A) It was the same on average.
B) 3° to 8° C warmer
C) 3° to 8° C colder
D) 10° to 20°C colder
E) 20° to 30°C colder
A) It was the same on average.
B) 3° to 8° C warmer
C) 3° to 8° C colder
D) 10° to 20°C colder
E) 20° to 30°C colder

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
46
There are a number of layers in the atmosphere where oxygen molecules are broken into atoms that can combine with other O2 molecules to make ozone (O3). Why is it that there is only a specific region/layer where ozone is made in any significant amount?
A) The reaction is faster at higher altitudes.
B) The concentration of O2 is not large enough at higher altitudes.
C) The energy from the sun is not strong enough at higher altitudes.
D) The molecules cannot breathe at higher altitudes.
E) The reaction is slow at higher altitudes.
A) The reaction is faster at higher altitudes.
B) The concentration of O2 is not large enough at higher altitudes.
C) The energy from the sun is not strong enough at higher altitudes.
D) The molecules cannot breathe at higher altitudes.
E) The reaction is slow at higher altitudes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
47
How would you explain to a friend the size of the nucleus of an atom versus the size of the entire atom?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
48
How many electrons are present in 8.0 grams of He?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many protons are in 3.5 moles of carbon atoms?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
50
The density of lead is 11.34 g/cm3; if the lead nucleus is 1/100 000 times that of the atom, determine the density of the nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
51
The density of gold is 19.3 g cm-3. Determine the radius of a single gold atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
52
Magnesium has a binding energy of 258.6 kJ/mole. If filtered light from a mercury lamp with wavelength 383 nm is absorbed by magnesium metal, what is the maximum kinetic energy of electrons observed?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
53
Platinum is a quite inert metal used in many ways, recently popular in jewelry. It has a binding energy of 513 kJ/mole. What is the longest wavelength of electromagnetic radiation possible that will have enough energy to eject photoelectrons from a platinum atom?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
54
Lasers are commonly used to mark and cut metals with high precision. One industrial laser is the neodymium-YAG laser that produces laser light of wavelength 1065 nm. If you want to vaporize 50 mg of Pt, which requires about 250 J of energy, how many photons of YAG laser light are needed?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
55
The ruby laser contains chromium (III) ions in Al2O3. It is the Cr(III) ion that emits light of 627 nm under the laser emission conditions. If a ruby contains 0.0010% Cr by mass, what is the minimum mass of a ruby (in g) required to produce a single pulse with energy 1.5 J?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
56
A glass filled with water absorbs 2.5 x 1020 photons of wavelength 1500 nm. How much energy is transferred to the water?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
57
An electron on a hydrogen atom in the outer atmosphere of a star can be excited to very high quantum numbers. What is the wavelength of light emitted when a hydrogen atom undergoes a transition from n = 1000 to n = 999?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
58
Hydrogen atoms are present for fleeting amounts of time in combustion processes. They can be observed by monitoring the amount of light absorbed at the wavelength corresponding to the n = 2 to n = 3 transition. What is the wavelength of this electromagnetic radiation and in what region of the spectrum does it lie?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
59
The energy levels of the dication of lithium, Li2+, are described by a modified Bohr equation; all energy levels are nine times those of the H atom. What is the wavelength (in nm) emitted by n Li2+ ion going from the n = 5 to n = 4 energy level?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
60
A number of scientists, when they saw the hydrogen gas emission lines, thought that the electrons in the hydrogen atom could have any energy and that they were only seeing a few of those energies. How did Bohr interpret these emission lines and why was this a new or unique interpretation?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
61
Show the path of an electron on an atom as it absorbs energy going from energy level 1 to level 6 and then releasing energy (as a photon) to fall to energy level 3. Be sure to show the relative differences in energy of the levels.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
62
The photoelectric effect experiment is performed on strontium metal. When light with ? = 389 nm shines on the metal, electrons are ejected at a velocity of 3.97 x 105 m/s. What is the threshold frequency, o, for strontium metal?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the wavelength of electrons moving at 1.5 x106 m/s (about 0.5% the speed of light)?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the kinetic energy in kJ/mole of electrons with a wavelength of 0.22 nm (the distance between some atoms)?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the wavelength of electrons with kinetic energy 95 kJ/mole?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
66
At what velocity would an electron need to travel at to have a wavelength of 0.1 nm?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
67
An electron has velocity of 3 x 102 m s-1; determine the wavelength and frequency of this electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
68
One wavelength of infrared radiation absorbed by water is 3150 nm. At what velocity will electrons have this wavelength?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
69
List the possible values of ml for an electron in a 3p subshell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
70
List the possible values for l for an electron in the 4th principle quantum level.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
71
List all possible quantum sets for an electron in the 4p quantum level.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
72
Atomic orbitals on different atoms can interact with each other, with different results. For now, consider two atoms, A and X. Draw contour pictures of the interaction of the 2s orbital on A with the 2p orbital on X, with the preferred axis of the p orbital (one of the lobes) pointing at the s orbital.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
73
Atomic orbitals on different atoms can interact with each other, with different results. For now, consider two atoms, A and X. Draw contour pictures of the 2p orbital on A and the 2p orbital on X where the preferred axes are parallel (a side-by-side interaction).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following pictures shows the appropriate number of nodes for a 3d orbital? 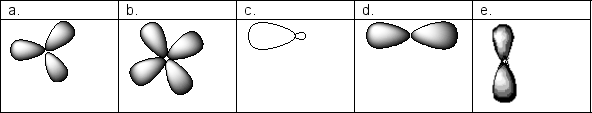


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which of the following is a view of the dxy orbital from the z axis? 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
76
Please define what a node is and how many would be found in a 4p orbital.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
77
The energy required to convert O2 molecules to O atoms is 496 kJ/mole. If electromagnetic radiation of 180 nm is absorbed by 1 mole of O2 molecules, how much kinetic energy will be present in the O atoms?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
78
Draw a molecular picture illustrating the decomposition of O3 to O2 + O in the stratosphere.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
79
The bond energy for molecular oxygen, O2, is 495 kJ/mol; the maximum wavelength of light capable of fragmenting the ozone molecule is 340 nm. What is the difference between the bond energies of O2 and O3?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
80
The bond energy for molecular oxygen, O2, is 495 kJ/mol and differs from that of O3 by 145 kJ mol-1; what is the maximum wavelength of light capable of fragmenting the ozone molecule?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck








