Deck 9: Solids
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/31
العب
ملء الشاشة (f)
Deck 9: Solids
1
Which of the following types of solids represents the most ordered arrangement of repeating units?
A) polycrystalline
B) crystalline
C) hydrophobic
D) amorphous
E) none of these
A) polycrystalline
B) crystalline
C) hydrophobic
D) amorphous
E) none of these
crystalline
2
Diamond is an example of:
A) a molecular solid
B) a network covalent solid
C) an ionic solid
D) a solid solution
E) a metallic solid
A) a molecular solid
B) a network covalent solid
C) an ionic solid
D) a solid solution
E) a metallic solid
a network covalent solid
3
SiO2 is a network covalent solid. Which of the following is most likely to be true of SiO2?
A) SiO2 would have a low melting point and would conduct electricity when melted.
B) SiO2 would conduct electricity as a solid and its melting point cannot be predicted.
C) SiO2 would have a high melting point and would conduct electricity when melted.
D) SiO2 would have a high melting point and wouldn't conduct electricity when melted.
E) SiO2 would have a low melting point and wouldn't conduct electricity when melted.
A) SiO2 would have a low melting point and would conduct electricity when melted.
B) SiO2 would conduct electricity as a solid and its melting point cannot be predicted.
C) SiO2 would have a high melting point and would conduct electricity when melted.
D) SiO2 would have a high melting point and wouldn't conduct electricity when melted.
E) SiO2 would have a low melting point and wouldn't conduct electricity when melted.
SiO2 would have a high melting point and wouldn't conduct electricity when melted.
4
Which of the following types of solids is most likely to be soft, have a low melting point, and not conduct electricity when melted?
A) ionic
B) molecular
C) network covalent
D) metallic
E) none of the above
A) ionic
B) molecular
C) network covalent
D) metallic
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which solid is held together by an extended network of covalent bonds?
A) sodium chloride
B) gold
C) calcium carbonate
D) diamond
E) dry ice (solid CO2)
A) sodium chloride
B) gold
C) calcium carbonate
D) diamond
E) dry ice (solid CO2)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which force makes the most important contribution to the lattice energy of solid CO2?
A) metallic bonding
B) ionic bonding
C) hydrogen bonding
D) dispersion forces
E) all of the above
A) metallic bonding
B) ionic bonding
C) hydrogen bonding
D) dispersion forces
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
7
Diamond (C(s)) is a network covalent solid. Which of the following is most likely to be true of diamond?
A) Diamond would have a low melting point and would conduct electricity when melted.
B) Diamond would conduct electricity as a solid and its melting point cannot be predicted.
C) Diamond would have a high melting point and would conduct electricity when melted.
D) Diamond would have a high melting point and wouldn't conduct electricity when melted.
E) Diamond would have a low melting point and wouldn't conduct electricity when melted.
A) Diamond would have a low melting point and would conduct electricity when melted.
B) Diamond would conduct electricity as a solid and its melting point cannot be predicted.
C) Diamond would have a high melting point and would conduct electricity when melted.
D) Diamond would have a high melting point and wouldn't conduct electricity when melted.
E) Diamond would have a low melting point and wouldn't conduct electricity when melted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
8
A compound that is a poor conductor of electricity when solid, but a very good conductor when molten is most likely to fit into which category?
A) molecular solid
B) covalent solid
C) ionic solid
D) metallic solid
E) any of the above
A) molecular solid
B) covalent solid
C) ionic solid
D) metallic solid
E) any of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
9
Arrange the following ionic compounds in order of increasing lattice energy (energy to break the solid into its gaseous ions).
MgF2, SrF2, BeF2
A) MgF2 < SrF2 < BeF2
B) SrF2 < MgF2 < BeF2
C) MgF2 < BeF2 < SrF2
D) SrF2 < BeF2 < MgF2
E) BeF2 < MgF2 < SrF2
MgF2, SrF2, BeF2
A) MgF2 < SrF2 < BeF2
B) SrF2 < MgF2 < BeF2
C) MgF2 < BeF2 < SrF2
D) SrF2 < BeF2 < MgF2
E) BeF2 < MgF2 < SrF2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
10
Use the bond-type triangle for
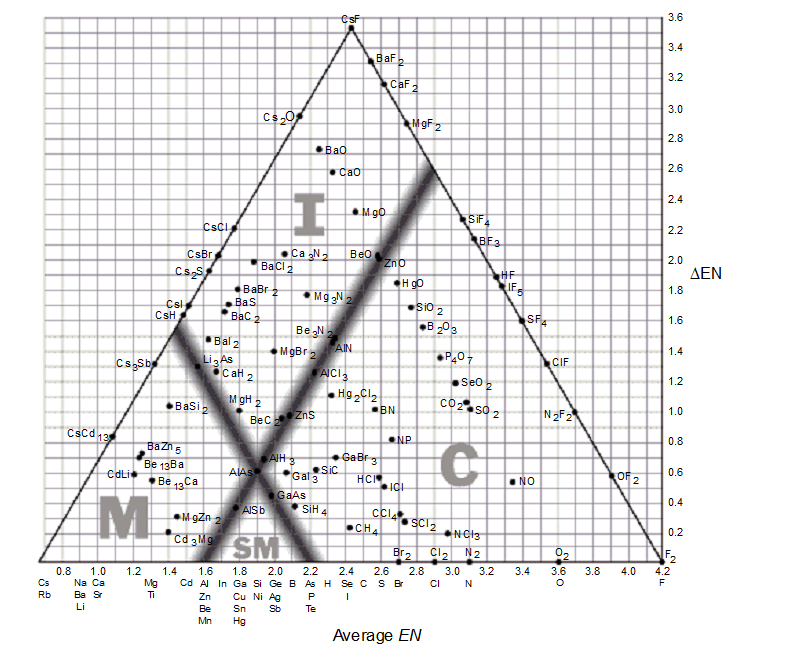
-Which of the following compounds will have the most ionic character?
A) AlSb
B) AlN
C) AlLi
D) AlP
E) AlAs

-Which of the following compounds will have the most ionic character?
A) AlSb
B) AlN
C) AlLi
D) AlP
E) AlAs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
11
Use the bond-type triangle for

-Which of the following compounds will have the most covalent character?
A) NaCl
B) SrCl2
C) PCl3
D) AlCl3
E) SnCl4

-Which of the following compounds will have the most covalent character?
A) NaCl
B) SrCl2
C) PCl3
D) AlCl3
E) SnCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
12
Use the bond-type triangle for

-Which of the following compounds will have the most metallic character?
A) SnBr4
B) SnO2
C) SnP
D) SnS
E) SnCl4

-Which of the following compounds will have the most metallic character?
A) SnBr4
B) SnO2
C) SnP
D) SnS
E) SnCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
13
Use the bond-type triangle for

-Predict which compound has a high melting point and is a conductor in the molten and aqueous state.
A) AlSb
B) AlN
C) AlLi
D) AlP
E) AlAs

-Predict which compound has a high melting point and is a conductor in the molten and aqueous state.
A) AlSb
B) AlN
C) AlLi
D) AlP
E) AlAs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
14
Use the bond-type triangle for

-Predict which compound is an electrical insulator and has a relatively low melting point.
A) NaIn
B) SrCl2
C) PCl3
D) AlCl3
E) CaH2

-Predict which compound is an electrical insulator and has a relatively low melting point.
A) NaIn
B) SrCl2
C) PCl3
D) AlCl3
E) CaH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
15
Use the bond-type triangle for

-Predict which solid is the best electrical conductor.
A) SnBr4
B) SnO2
C) SnP
D) SnS
E) SnCl4

-Predict which solid is the best electrical conductor.
A) SnBr4
B) SnO2
C) SnP
D) SnS
E) SnCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
16
Use the bond-type triangle for

-Predict which compound is an insulating ceramic material.
A) MgZn2
B) Li3P
C) Li2O
D) LiCl
E) GaAs

-Predict which compound is an insulating ceramic material.
A) MgZn2
B) Li3P
C) Li2O
D) LiCl
E) GaAs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following structures has a coordination number of six?
A) simple cubic
B) body-centered cubic
C) cubic closest packed
D) hexagonal closest packed
E) none of the above
A) simple cubic
B) body-centered cubic
C) cubic closest packed
D) hexagonal closest packed
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following correctly describes a body-centered cubic structure?
A) Coordination Number = 6, 1 atom per unit cell
B) Coordination Number = 6, 2 atoms per unit cell
C) Coordination Number = 6, 4 atoms per unit cell
D) Coordination Number = 8, 1 atom per unit cell
E) Coordination Number = 8, 2 atoms per unit cell
A) Coordination Number = 6, 1 atom per unit cell
B) Coordination Number = 6, 2 atoms per unit cell
C) Coordination Number = 6, 4 atoms per unit cell
D) Coordination Number = 8, 1 atom per unit cell
E) Coordination Number = 8, 2 atoms per unit cell

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the ratio of the length of the body diagonal of a cube to the face diagonal of the cube?
A) 0.817
B) 1.00
C) 1.22
D) 1.41
E) 1.73
A) 0.817
B) 1.00
C) 1.22
D) 1.41
E) 1.73

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the relationship between the unit cell edge length (a) and the radius of a metal atom (r) when the metal crystallizes in a face-centered cubic unit cell?
A) r =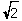 a
a
B) r = (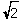 a)/4
a)/4
C) r = (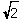 a)/2
a)/2
D)r = (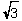 a)/4
a)/4
E) none of the above
A) r =
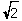 a
aB) r = (
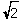 a)/4
a)/4C) r = (
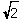 a)/2
a)/2D)r = (
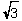 a)/4
a)/4E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
21
Calcium titanate crystallizes in a cubic unit cell with titanium atoms at the corners of the cell, oxygen atoms in the middle of the faces of the cell, and a calcium atom in the center of the cell. What is the formula of calcium titanate?
A) CaTiO
B) CaTiO3
C) CaTiO4
D) Ca2TiO4
E) CaTi8O2
A) CaTiO
B) CaTiO3
C) CaTiO4
D) Ca2TiO4
E) CaTi8O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
22
Calculate the number of chloride and ammonium ions per unit cell if NH4Cl is a simple cubic unit cell of NH4+ ions with a Cl- ion in the center of the unit cell.
A) 1 NH4+ ion and 1 Cl- ion
B) 2 NH4+ ions and 2 Cl- ions
C) 4 NH4+ ions and 4 Cl- ions
D) 8 NH4+ ions and 8 Cl- ions
E) none of the above
A) 1 NH4+ ion and 1 Cl- ion
B) 2 NH4+ ions and 2 Cl- ions
C) 4 NH4+ ions and 4 Cl- ions
D) 8 NH4+ ions and 8 Cl- ions
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the density in g/cm3 of sodium if this metal crystallizes in a body-centered cubic unit cell with a cell edge of 0.429 nm?
A) 0.484 g/cm3
B) 0.582 g/cm3
C) 0.967 g/cm3
D) 1.94 g/cm3
E) 9.67 x 10-25 g/cm3
A) 0.484 g/cm3
B) 0.582 g/cm3
C) 0.967 g/cm3
D) 1.94 g/cm3
E) 9.67 x 10-25 g/cm3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the approximate density in g/cm3 of titanium metal if the atomic radius of a titanium atom is 0.145 nm and the atoms form a body-centered cubic unit cell?
A) 0.1 g/cm3
B) 1 g/cm3
C) 5 g/cm3
D) 25 g/cm3
E) 30 g/cm3
A) 0.1 g/cm3
B) 1 g/cm3
C) 5 g/cm3
D) 25 g/cm3
E) 30 g/cm3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
25
Tungsten crystallizes in a cubic unit cell with a cell edge of 0.3981 nm. The density of tungsten is 19.35 g/cm3. Calculate the number of atoms per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
26
Iron crystallizes in a cubic unit cell with a cell edge of 0.2866 nm. The density of iron is 7.875 g/cm3. Calculate the number of atoms per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
27
Calculate the metallic radius in nanometers of the Ag atom if silver crystallizes in a face-centered cubic unit cell with a cell edge of 0.4086 nm.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
28
Cesium iodide consists of a simple cubic lattice of l- ions with Cs+ ions in the center of the cubes. If the cell edge length is 0.445 nm, what is the Cs-I interatomic distance in nanometers?
A) 0.193 nm
B) 0.314 nm
C) 0.385 nm
D) 0.629 nm
E) 0.770 nm
A) 0.193 nm
B) 0.314 nm
C) 0.385 nm
D) 0.629 nm
E) 0.770 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
29
In KF, the K+ and F- ions are almost exactly the same size: 0.134 nm. Which would be larger: a neutral potassium atom or a neutral fluorine atom?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following would be the correct order from lowest melting point to highest for the following substances?
A) NaF < C3H8 < CH3CH2OH
B) C3H8 < NaF < CH3CH2OH
C) C3H8 < CH3CH2OH < NaF
D) CH3CH2OH < C3H8 < NaF
E) none of these
A) NaF < C3H8 < CH3CH2OH
B) C3H8 < NaF < CH3CH2OH
C) C3H8 < CH3CH2OH < NaF
D) CH3CH2OH < C3H8 < NaF
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
31
When diamond, NaCl, and water are boiled, the bonds being broken can best be described respectively as:
A) covalent, ionic, covalent
B) ionic, covalent, hydrogen bonds
C) covalent, ionic, hydrogen bonds
D) hydrogen bonds, ionic, covalent
E) none of the above
A) covalent, ionic, covalent
B) ionic, covalent, hydrogen bonds
C) covalent, ionic, hydrogen bonds
D) hydrogen bonds, ionic, covalent
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck








