Deck 3: Structure of the Atom
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/106
العب
ملء الشاشة (f)
Deck 3: Structure of the Atom
1
What would you describe as the key result of the Rutherford experiment, in which a metal target was bombarded with
-particles?
A) The -particles were able to pass through the target.
B) The particles were found to knock electrons out of the target.
C) Some of the -particles were deflected through large angles.
D) Flashes of light were emitted when the -particles hit the ZnS screen after they passed through the target.
E) The experiment showed the need for better -particle detectors.
-particles?
A) The -particles were able to pass through the target.
B) The particles were found to knock electrons out of the target.
C) Some of the -particles were deflected through large angles.
D) Flashes of light were emitted when the -particles hit the ZnS screen after they passed through the target.
E) The experiment showed the need for better -particle detectors.
Some of the -particles were deflected through large angles.
2
O2 molecules can dissociate to form O atoms by absorbing electromagnetic radiation. If it takes 498 kJ to dissociate one mole of O2 molecules to form two moles of O atoms, what is the frequency of the light that would have just enough energy to decompose a single O2 molecule into O atoms?
A) 1.25 x 1015 s-1
B) 8.27 x 10-19 s-1
C) 7.52 x 1038 s-1
D) 8.0 x 10-16 s-1
E) 5.72 x 1016 s-1
A) 1.25 x 1015 s-1
B) 8.27 x 10-19 s-1
C) 7.52 x 1038 s-1
D) 8.0 x 10-16 s-1
E) 5.72 x 1016 s-1
1.25 x 1015 s-1
3
O2 molecules can dissociate to form O atoms by absorbing electromagnetic radiation. If it takes 498 kJ to dissociate one mole of O2 molecules to form two moles of O atoms, in what portion of the electromagnetic spectrum would light have sufficient energy to cause this reaction to occur?
A) radio wave
B) microwave
C) infrared
D) visible
E) ultraviolet
A) radio wave
B) microwave
C) infrared
D) visible
E) ultraviolet
ultraviolet
4
In a 23.490 kilogauss magnetic field, 13C nuclei absorb electromagnetic radiation in the RF (radio) portion of the spectrum at a frequency of 25.147 MHz (25.147 x 106 s-1.) Calculate the wavelength of this radiation.
A) longer than 10 meters
B) between 10 and 0.10 meters
C) between 0.10 and 10-4 meters
D) between 10-4 and 10-6
E) shorter than 10-6 meters
A) longer than 10 meters
B) between 10 and 0.10 meters
C) between 0.10 and 10-4 meters
D) between 10-4 and 10-6
E) shorter than 10-6 meters

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the wavelength in centimeters of light that has a frequency of 2.33 x 1015 s-1?
A) 6.99 x 1025 cm
B) 7.77 x 104 cm
C) 1.290 x 103 cm
D) 1.29 x 10-5 cm
E) none of the above
A) 6.99 x 1025 cm
B) 7.77 x 104 cm
C) 1.290 x 103 cm
D) 1.29 x 10-5 cm
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
6
A helium-neon laser emits light with a wavelength of 6328 Å. What is the energy of a 6328 Å photon in joules?
A) 2.179 x 10-19 J
B) 3.139 x 10-19 J
C) 5.448 x 10-19 J
D) 6.328 x 10-19 J
E) 2.179 x 10-18 J
A) 2.179 x 10-19 J
B) 3.139 x 10-19 J
C) 5.448 x 10-19 J
D) 6.328 x 10-19 J
E) 2.179 x 10-18 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
7
If green light has a frequency of 5.0 x 1014 s-1, what is the wavelength (in meters) of this light?
A) 6.7 x 10-24
B) 2.0 x 10-15
C) 6.0 x 10-7
D) 3.0 x 108
E) none of the above
A) 6.7 x 10-24
B) 2.0 x 10-15
C) 6.0 x 10-7
D) 3.0 x 108
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
8
If X-rays have a shorter wavelength than ultraviolet light, which of the following statements is true?
A) X-rays have smaller frequencies than UV light.
B) X-rays travel faster than UV light.
C) X-rays have more energy than UV light.
D) X-rays have larger amplitudes than UV light.
E) None of the above statements are true.
A) X-rays have smaller frequencies than UV light.
B) X-rays travel faster than UV light.
C) X-rays have more energy than UV light.
D) X-rays have larger amplitudes than UV light.
E) None of the above statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
9
There is a fundamental difference between the long wavelength IR radiation given off by the toy ovens sold at Toys 'R Us and the shorter wavelength UV radiation emitted by the tanning booths at your local strip mall. Which of the following statements is
A) IR radiation has a higher frequency than UV radiation.
B) IR radiation carries more energy per photon than UV radiation.
C) IR radiation carries a larger amplitude than UV radiation.
D) IR radiation travels slower than UV radiation.
E) None of the above statements are true.
A) IR radiation has a higher frequency than UV radiation.
B) IR radiation carries more energy per photon than UV radiation.
C) IR radiation carries a larger amplitude than UV radiation.
D) IR radiation travels slower than UV radiation.
E) None of the above statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
10
A cheap spectrophotometer can be made using a light-emitting diode (LED) as the source of the light. Assume that you build one of these spectrometers using an LED that gives off green light with a wavelength of 520 nm. What is the energy of a photon of this green light?
A) 1.15 x 10-34 J
B) 1.03 x 10-22 J
C) 3.82 x 10-21 J
D) 3.82 x 10-19 J
E) none of the above
A) 1.15 x 10-34 J
B) 1.03 x 10-22 J
C) 3.82 x 10-21 J
D) 3.82 x 10-19 J
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
11
Water ionizes according to the following reaction which requires approximately 1200 kJ/mol of energy
H2O(l) + h v H2O+ + e-
In what portion of the electromagnetic spectrum are you likely to find the radiation that carries just enough energy to be dangerous to life because it can ionize the water that is so important to living organisms?
A) Radio/TV waves, 10 - 0.1 m
B) Microwaves, 0.01 - 10-4 m
C) Ultraviolet, 10-7 - 10-9 m
D) X-rays, 10-10 - 10-12 m
E) -rays, 10-12 - 10-14 m
H2O(l) + h v H2O+ + e-
In what portion of the electromagnetic spectrum are you likely to find the radiation that carries just enough energy to be dangerous to life because it can ionize the water that is so important to living organisms?
A) Radio/TV waves, 10 - 0.1 m
B) Microwaves, 0.01 - 10-4 m
C) Ultraviolet, 10-7 - 10-9 m
D) X-rays, 10-10 - 10-12 m
E) -rays, 10-12 - 10-14 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following wavelengths or frequencies of light will have photons with the highest energy?
A) 450 nm
B) 4.5 x 1014 Hz
C) 600 nm
D) 5.0 x 1014 Hz
E) none of these
A) 450 nm
B) 4.5 x 1014 Hz
C) 600 nm
D) 5.0 x 1014 Hz
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
13
Yellow light has a frequency of 5.2 x 1014 cycles per second while red light has a frequency of 4.3 x 1014 cycles per second. Which of the following correctly describes the relationship between the wavelength and energy of yellow versus red light?
A) Red light has a longer wavelength and more energy than yellow light.
B) Yellow light has a longer wavelength and more energy than red light.
C) Red light has a longer wavelength and less energy than yellow light.
D) Yellow light has a longer wavelength and less energy than red light.
E) None of these.
A) Red light has a longer wavelength and more energy than yellow light.
B) Yellow light has a longer wavelength and more energy than red light.
C) Red light has a longer wavelength and less energy than yellow light.
D) Yellow light has a longer wavelength and less energy than red light.
E) None of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
14
Photochemistry is when light causes a chemical reaction to occur. A simple example is when light breaks a Cl2 molecule apart into 2 Cl atoms. If the energy required to break apart a mole of chlorine molecules is 243 kJ, a photon of what frequency will break apart a single Cl2 molecule?
A) 6.1 x 1014 s-1
B) 4.0 x 10-19 s-1
C) 3.7 x 1035 s-1
D) 5.2 x 1015 s-1
E) none of the above.
A) 6.1 x 1014 s-1
B) 4.0 x 10-19 s-1
C) 3.7 x 1035 s-1
D) 5.2 x 1015 s-1
E) none of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
15
A recent article in Scientific American notes that life would be impossible on any planet close enough to the Sun to boil water, or far enough from the Sun that water froze. It also argues that the Earth is in one of the few parts of the galaxy where life could exist. Too close to the core, and either collisions with other objects would destroy the planet or cosmic radiation from neighboring stars would destroy life. Too far from the sun and there wouldn't be enough of the elements needed to form a planet. Let's assume that radiation becomes particularly dangerous to life when it carries enough energy to ionize a water molecule when it is absorbed. ?

Use Avogadro's number and Planck's constant to calculate the frequency of this radiation to one significant figure. (Hint: Pay close attention to units!)
A) 3 x 109 s-1
B) 3 x 1012 s-1
C) 3 x 1015 s-1
D) 3 x 1018 s-1
E) 3 x 1021 s-1

Use Avogadro's number and Planck's constant to calculate the frequency of this radiation to one significant figure. (Hint: Pay close attention to units!)
A) 3 x 109 s-1
B) 3 x 1012 s-1
C) 3 x 1015 s-1
D) 3 x 1018 s-1
E) 3 x 1021 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
16
In 1814 Fraunhofer observed a series of dark lines in the sun's spectrum, which he labeled A through H. About 50 years later, Gustav Kirchhoff noticed that the wavelength of light given off when sodium salts are added to a flame is the same as the wavelength of the D line in Fraunhofer's spectrum. He concluded that certain substances give off light when heated that has the same wavelength as the light absorbed under other conditions. The wavelength of the characteristic yellow-orange light emitted by sodium ions in a burner flame is 589.5923 nm. What is the energy of this light, in units of kJ/mol?
A) less than 1 kJ/mol
B) between 1 and 10 kJ/mol
C) between 10 and 100 kJ/mol
D) between 100 and 1000 kJ/mol
E) more than 1000 kJ/mol
A) less than 1 kJ/mol
B) between 1 and 10 kJ/mol
C) between 10 and 100 kJ/mol
D) between 100 and 1000 kJ/mol
E) more than 1000 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
17
What do we mean when we say that "The energy of the electron in an atom is quantized?"
A) The electron has a very small amount of energy.
B) The energy of the electron is proportional to the mass of the nucleus.
C) When an electron changes its energy, it emits a quantum of light.
D) The energy of the electron can have certain fixed energies and not others.
E) The electron must be a wave.
A) The electron has a very small amount of energy.
B) The energy of the electron is proportional to the mass of the nucleus.
C) When an electron changes its energy, it emits a quantum of light.
D) The energy of the electron can have certain fixed energies and not others.
E) The electron must be a wave.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
18
The line structure of the emission spectrum of the hydrogen atom suggests that:
A) The atom is composed of a small positive nucleus and electrons.
B) There are many electrons in a hydrogen atom.
C) The position and energy of an electron cannot be determined simultaneously.
D) The energies of electrons are quantized.
E) Each electron has multiple energies.
A) The atom is composed of a small positive nucleus and electrons.
B) There are many electrons in a hydrogen atom.
C) The position and energy of an electron cannot be determined simultaneously.
D) The energies of electrons are quantized.
E) Each electron has multiple energies.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following statements about the Bohr model is wrong?
A) The energy of the electron is quantized.
B) All possible wavelengths can occur in the emission spectrum since n runs from 1 to infinity.
C) The angular momentum of the electron is quantized.
D) All quantized energies are negative.
E) The electron travels around the nucleus in an orbit.
A) The energy of the electron is quantized.
B) All possible wavelengths can occur in the emission spectrum since n runs from 1 to infinity.
C) The angular momentum of the electron is quantized.
D) All quantized energies are negative.
E) The electron travels around the nucleus in an orbit.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following transitions in the spectrum of the hydrogen atom results in the absorption of a photon with the largest energy?
A) n = 2 to n = 3
B) n = 2 to n = 4
C) n = 1 to n = 4
D) n = 3 to n = 1
E) n = 7 to n = 1
A) n = 2 to n = 3
B) n = 2 to n = 4
C) n = 1 to n = 4
D) n = 3 to n = 1
E) n = 7 to n = 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which transition shown below will require the absorption of the shortest wavelength photon?
A) n = 1 to n = 3
B) n = 2 to n = 3
C) n = 1 to n = 5
D) n = 8 to n = 1
E) All of the above will require the same wavelength photon.
A) n = 1 to n = 3
B) n = 2 to n = 3
C) n = 1 to n = 5
D) n = 8 to n = 1
E) All of the above will require the same wavelength photon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which transition in the spectrum of the hydrogen atom results in the emission of light with the longest wavelength?
A) n = 3 to n = 2
B) n = 3 to n = 1
C) n = 5 to n = 4
D) n = 2 to n = 3
E) n = 1 to n = 3
A) n = 3 to n = 2
B) n = 3 to n = 1
C) n = 5 to n = 4
D) n = 2 to n = 3
E) n = 1 to n = 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the energy of the light emitted when an electron falls from the n = 4 to the n = 2 orbit in the hydrogen atom?
A) 4.09 x 10-19 J
B) 5.45 x 10-18 J
C) 4.36 x 10-18 J
D) 2.62 x 10-17 J
E) none of the above
A) 4.09 x 10-19 J
B) 5.45 x 10-18 J
C) 4.36 x 10-18 J
D) 2.62 x 10-17 J
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
24
If a laser operated on the n = 5 to n = 2 transition of a H atom, what wavelength in nm photon would be emitted?
A) 380 nm
B) 434 nm
C) 1950 nm
D) 3910 nm
E) none of the above
A) 380 nm
B) 434 nm
C) 1950 nm
D) 3910 nm
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
25
Arrange the following elements in order of increasing first ionization energy:
S, Ar, Ca
A) S < Ar < Ca
B) Ar < S < Ca
C) Ca < Ar < S
D) S < Ca < Ar
E) Ca < S < Ar
S, Ar, Ca
A) S < Ar < Ca
B) Ar < S < Ca
C) Ca < Ar < S
D) S < Ca < Ar
E) Ca < S < Ar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
26
Why is the ionization energy of potassium (K) less than the ionization energy of sodium (Na)?
A) The ionized electron in K is in a higher energy shell than the ionized electron in Na.
B) Because the core charge remains the same going down a group
C) Because K has more protons in its nucleus than Na.
D) Because the core charge decreases down a group
E) Because the ionized electron in K is in a higher energy shell AND because the core charge of K and Na are the same.
A) The ionized electron in K is in a higher energy shell than the ionized electron in Na.
B) Because the core charge remains the same going down a group
C) Because K has more protons in its nucleus than Na.
D) Because the core charge decreases down a group
E) Because the ionized electron in K is in a higher energy shell AND because the core charge of K and Na are the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
27
The first ionization energy of the first six elements in the periodic table is given below.

Why is the first ionization energy of B smaller than Be?
A) Because the first IE decreases as we go across a row of the periodic table from left to right.
B) Because the nucleus of a B (Z = 5) atom contains fewer protons than the nucleus of a Be (Z = 4) atom.
C) Because the outermost electron on B is coming from a 2p, not a 2s orbital.
D) Because the atomic number of B is odd, whereas the atomic number of Be is even.
E) For the same reason that the first IE of He is larger than that of H.

Why is the first ionization energy of B smaller than Be?
A) Because the first IE decreases as we go across a row of the periodic table from left to right.
B) Because the nucleus of a B (Z = 5) atom contains fewer protons than the nucleus of a Be (Z = 4) atom.
C) Because the outermost electron on B is coming from a 2p, not a 2s orbital.
D) Because the atomic number of B is odd, whereas the atomic number of Be is even.
E) For the same reason that the first IE of He is larger than that of H.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
28
Arrange the following elements in order of increasing first ionization energy:
Ne, Na, Mg
A) Ne < Na < Mg
B) Na < Mg < Ne
C) Ne < Mg < Na
D) Mg < Ne < Na
E) Na < Ne < Mg
Ne, Na, Mg
A) Ne < Na < Mg
B) Na < Mg < Ne
C) Ne < Mg < Na
D) Mg < Ne < Na
E) Na < Ne < Mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
29
Construct a shell model for potassium, K, like Figure 3.9 in the text.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
30
Construct a shell model for calcium, Ca, like Figure 3.9 in the text.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
31
What is the core charge for the potassium atom, K?
A) +1
B) +2
C) +8
D) +9
E) +19
A) +1
B) +2
C) +8
D) +9
E) +19

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following provides the best evidence for the presence of the electron shell structure of atoms?
A) The decrease in ionization energy going down a group.
B) The increase in ionization energy across a period.
C) That the value of ms can be +1/2 or -1/2
D) That the ionization energy of O is less than the ionization energy of N.
E) All of the above provide evidence for the shell structure of atoms.
A) The decrease in ionization energy going down a group.
B) The increase in ionization energy across a period.
C) That the value of ms can be +1/2 or -1/2
D) That the ionization energy of O is less than the ionization energy of N.
E) All of the above provide evidence for the shell structure of atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the core charge for the calcium atom, Ca?
A) +1
B) +2
C) +8
D) +10
E) +20
A) +1
B) +2
C) +8
D) +10
E) +20

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the core charge for the silicon atom, Si?
A) +1
B) +2
C) +4
D) +6
E) +8
A) +1
B) +2
C) +4
D) +6
E) +8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
35
How would you expect the first ionization energy of K to compare to that of Ar?
A) K > Ar
B) K = Ar
C) K < Ar
D) First ionization energies for an atom can vary
E) Ar does not ionize
A) K > Ar
B) K = Ar
C) K < Ar
D) First ionization energies for an atom can vary
E) Ar does not ionize

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
36
Arrange the following atoms in order of decreasing energy needed to remove the most tightly held electron (the electron closest to the nucleus).
Na, Mg, Al, P
A) Na > Mg > Al > P
B) Na > Mg > P > Al
C) Al > P > Na > Mg
D) Al > P > Mg > Na
E) P > Al > Mg > Na
Na, Mg, Al, P
A) Na > Mg > Al > P
B) Na > Mg > P > Al
C) Al > P > Na > Mg
D) Al > P > Mg > Na
E) P > Al > Mg > Na

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
37
Why is the first ionization energy of Br less than that of Cl?
A) Bromine has more protons than chlorine.
B) Bromine has more electrons than chlorine.
C) The core charge on bromine is less than that on chlorine.
D) The outer shell electrons in bromine are farther from the nucleus than in chlorine.
E) none of these
A) Bromine has more protons than chlorine.
B) Bromine has more electrons than chlorine.
C) The core charge on bromine is less than that on chlorine.
D) The outer shell electrons in bromine are farther from the nucleus than in chlorine.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
38
Predict the order of decreasing first ionization energies for Ar, K, and Ca.
A) Ar > K > Ca
B) Ca > K > Ar
C) K > Ar > Ca
D) K > Ca > Ar
E) Ar > Ca > K
A) Ar > K > Ca
B) Ca > K > Ar
C) K > Ar > Ca
D) K > Ca > Ar
E) Ar > Ca > K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which atom is represented by a shell model with two electrons in the first shell, and five electrons in the second shell?
A) Be
B) C
C) N
D) K
E) P
A) Be
B) C
C) N
D) K
E) P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following atoms has a core charge of +6?
A) B
B) C
C) N
D) Si
E) S
A) B
B) C
C) N
D) Si
E) S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which atom requires the largest amount of energy to remove the most tightly held electron (the electron closest to the nucleus)?
A) K
B) Ca
C) Ga
D) As
E) S
A) K
B) Ca
C) Ga
D) As
E) S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
42
Arrange the following atoms in order of increasing first ionization energies.
A) Be < Mg < Ca < Sr
B) Mg < Ca < Sr < Be
C) Sr < Ca < Mg < Be
D) Ca < Sr < Mg < Be
E) none of these are in the correct order
A) Be < Mg < Ca < Sr
B) Mg < Ca < Sr < Be
C) Sr < Ca < Mg < Be
D) Ca < Sr < Mg < Be
E) none of these are in the correct order

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
43
Arrange the following atoms in order of increasing first ionization energy.
A) P < S < Cl
B) Cl < S < P
C) S < P < Cl
D) S < Cl < P
E) Cl < P < S
A) P < S < Cl
B) Cl < S < P
C) S < P < Cl
D) S < Cl < P
E) Cl < P < S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
44
In which of the following groups of atoms does each atom have the same number of outer shell electrons?
A) B, C, N
B) K, Ca, P
C) C, Si, Ge
D) O, F, Cl
E) N, P, Cl
A) B, C, N
B) K, Ca, P
C) C, Si, Ge
D) O, F, Cl
E) N, P, Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
45
A photoelectron spectrum has three peaks. The first two are one third the size of the last one. Which atom corresponds to this spectrum?
A) He
B) Be
C) C
D) O
E) Ne
A) He
B) Be
C) C
D) O
E) Ne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
46
How do Photoelectron spectroscopy experiments differ from experiments used to obtain first ionization energies?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which element is represented by the following PES spectrum? 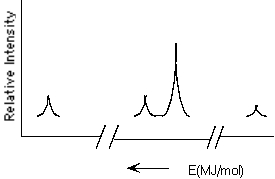
A) N
B) F
C) Na
D) Al
E) O

A) N
B) F
C) Na
D) Al
E) O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which element is represented by the following PES spectrum?
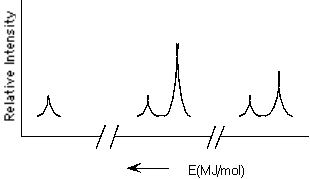
A) F
B) Si
C) S
D) K
E) Ti

A) F
B) Si
C) S
D) K
E) Ti

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which element is represented by the following PES spectrum?
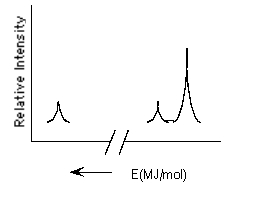
A) Li
B) Mg
C) Ca
D) Be
E) Ne

A) Li
B) Mg
C) Ca
D) Be
E) Ne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following atoms would have four peaks in its PES spectrum?
A) Li
B) Mg
C) F
D) K
E) O
A) Li
B) Mg
C) F
D) K
E) O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which is a legitimate set of n, l, ml and ms quantum numbers?
A) 0,0,0,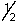
B) 8,4,-3,-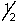
C) 3,3,2,+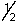
D) 2,1,-2,-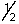
E) 5,3,3,-1
A) 0,0,0,
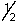
B) 8,4,-3,-
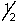
C) 3,3,2,+
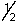
D) 2,1,-2,-
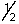
E) 5,3,3,-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which set of n, l, ml and ms quantum numbers is allowed?
A) 4,-2,-1,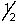
B) 4,2,3,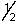
C) 4,3,0,1
D) 4,0,0,-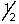
E) 4,2,2,2
A) 4,-2,-1,
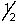
B) 4,2,3,
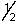
C) 4,3,0,1
D) 4,0,0,-
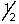
E) 4,2,2,2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following sets of n, l, ml and ms quantum numbers isn't allowed?
A) 1,1,0,+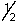
B) 2,0,0,+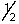
C) 3,2,1,-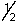
D) 4,1,-1,+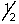
E) 5,3,2,-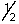
A) 1,1,0,+
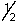
B) 2,0,0,+
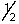
C) 3,2,1,-
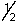
D) 4,1,-1,+
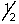
E) 5,3,2,-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which of the following selection rules for quantum numbers is incorrectly stated?
A) n is any integer greater than or equal to zero.
B) l is any integer between zero and n - 1.
C) ml is any integer between -/ and +/.
D) ms is either +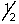 or -
or - 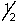 .
.
E) All of the above selection rules are correctly stated.
A) n is any integer greater than or equal to zero.
B) l is any integer between zero and n - 1.
C) ml is any integer between -/ and +/.
D) ms is either +
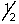 or -
or - 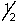 .
.E) All of the above selection rules are correctly stated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following quantum numbers is used to describe the orientation of an orbital in space?
A) n
B) /
C) ml
D) ms
E) None of these quantum numbers describe the orientation of an orbital in space.
A) n
B) /
C) ml
D) ms
E) None of these quantum numbers describe the orientation of an orbital in space.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which set of quantum numbers can be used to describe a 2p electron?
A) 2,1,0,-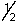
B) 2,0,0,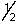
C) 2,2,1,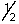
D) 3,2,1,-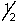
E) 3,1,0,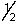
A) 2,1,0,-
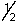
B) 2,0,0,
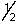
C) 2,2,1,
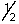
D) 3,2,1,-
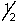
E) 3,1,0,
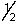

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
57
How many electrons can be placed in a 3d orbital?
A) 2
B) 6
C) 8
D) 10
E) 18
A) 2
B) 6
C) 8
D) 10
E) 18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of the following atomic orbitals doesn't exist?
A) 3f
B) 3p
C) 5f
D) 5d
E) 6s
A) 3f
B) 3p
C) 5f
D) 5d
E) 6s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
59
What is the orbital designation for the quantum numbers:
N = 4, l = 2, ml = -1?
A) 4s
B) 4p
C) 4d
D) 4f
E) none of these
N = 4, l = 2, ml = -1?
A) 4s
B) 4p
C) 4d
D) 4f
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
60
Orbitals for which l = 1 are described by which of the following symbols?
A) s
B) p
C) d
D) f
E) g
A) s
B) p
C) d
D) f
E) g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
61
What is the maximum number of unpaired electrons that can be accommodated in a 5d subshell?
A) 3
B) 5
C) 6
D) 7
E) 10
A) 3
B) 5
C) 6
D) 7
E) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the maximum number of electrons that can be accommodated in the subshell for which n = 3 and l = 2?
A) 2
B) 6
C) 10
D) 14
E) 18
A) 2
B) 6
C) 10
D) 14
E) 18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
63
Calculate the maximum number of electrons that can fit into the n = 4 shell of orbitals.
A) 6
B) 8
C) 18
D) 16
E) 32
A) 6
B) 8
C) 18
D) 16
E) 32

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
64
The Pauli exclusion principle states that:
A) No two electrons in an atom can have the same spin.
B) No two electrons in an atom can occupy the same orbital.
C) Two electrons in the same orbital have identical values of the spin quantum number.
D) No two electrons in an atom can have the same set of four quantum numbers.
E) Two electrons in an atom can have the same principal quantum number.
A) No two electrons in an atom can have the same spin.
B) No two electrons in an atom can occupy the same orbital.
C) Two electrons in the same orbital have identical values of the spin quantum number.
D) No two electrons in an atom can have the same set of four quantum numbers.
E) Two electrons in an atom can have the same principal quantum number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following quantum numbers can have a value that is not an integer?
A) n
B) l
C) ml
D) ms
E) None of the quantum numbers can have any value that is not an integer.
A) n
B) l
C) ml
D) ms
E) None of the quantum numbers can have any value that is not an integer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following subshells is filled first when electrons are added to the atomic orbitals on a xenon atom?
A) 4d
B) 4f
C) 5s
D) 5p
E) 5d
A) 4d
B) 4f
C) 5s
D) 5p
E) 5d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which of the following subshells would be filled first when electrons are added to a gold atom?
A) 4f
B) 5d
C) 6s
D) 6p
E) 6d
A) 4f
B) 5d
C) 6s
D) 6p
E) 6d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
68
Determine the group of the periodic table in which an element with the following electron configuration belongs: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
A) VA
B) IIIA
C) IVA
D) VIA
E) none of these
A) VA
B) IIIA
C) IVA
D) VIA
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
69
Determine the period and group in the periodic chart that contains the element with the following electron configuration:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
A) period 4, group VIA
B) period 6, group IVA
C) period 3, group IV B
D) period 4, group IVA
E) none of these
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
A) period 4, group VIA
B) period 6, group IVA
C) period 3, group IV B
D) period 4, group IVA
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
70
In what group of the periodic table would an element with the following electron configuration belong?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p1
A) Group IA
B) Group IlIA
C) Group VA
D) Group VIIA
E) none of the above
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p1
A) Group IA
B) Group IlIA
C) Group VA
D) Group VIIA
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
71
A single atom of element 109 has been synthesized. Predict the electronic configuration of this element. Which of the following elements would 109 most resemble?
A) Ta
B) Re
C) Ir
D) Au
E) Tl
A) Ta
B) Re
C) Ir
D) Au
E) Tl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
72
A single atom of element 114 was recently synthesized. Predict the electronic configuration of this element. Which of the following elements would 114 most resemble?
A) Au
B) Hg
C) Tl
D) Pb
E) Po
A) Au
B) Hg
C) Tl
D) Pb
E) Po

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
73
Theoreticians predict that the element with atomic number 120 will be more stable than the elements recently discovered with atomic numbers between 103 and 109. Based on the order of filling of atomic orbitals, the chemistry of this element should most closely resemble the chemistry of which of the following?
A) Ra, Group IIA
B) Pb, Group IVA
C) Po, Group VIA
D) Rn, Group VIIIA
E) one of the transition metals between La and Hg
A) Ra, Group IIA
B) Pb, Group IVA
C) Po, Group VIA
D) Rn, Group VIIIA
E) one of the transition metals between La and Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
74
In period 6 of the periodic table, the series of elements which differ in the number of electrons contained in f orbitals
A) begins with Cs and ends with Rn.
B) begins with La and ends with Hg.
C) begins with Tl and ends with Rn.
D) begins with La and ends with Rn.
E) begins with Ce and ends with Lu.
A) begins with Cs and ends with Rn.
B) begins with La and ends with Hg.
C) begins with Tl and ends with Rn.
D) begins with La and ends with Rn.
E) begins with Ce and ends with Lu.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
75
A possible set of quantum numbers for the last electron added to form a gallium atom (Z = 31) in its lowest energy state is:
A) 3, 1, 0, -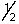
B) 3, 2, 1,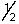
C) 4, 0, 0,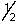
D) 4, 1, 1,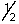
E) 4, 2, 2,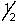
A) 3, 1, 0, -
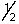
B) 3, 2, 1,
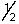
C) 4, 0, 0,
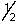
D) 4, 1, 1,
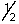
E) 4, 2, 2,
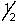

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
76
A possible set of quantum numbers for the highest energy electron in an As3+ ion is:
A) 3, 1, -1,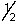
B) 4, 0, 0, -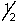
C) 3, 2, 0,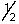
D) 4, 1, -1,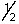
E) 5, 0, 0,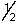
A) 3, 1, -1,
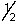
B) 4, 0, 0, -
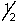
C) 3, 2, 0,
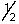
D) 4, 1, -1,
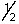
E) 5, 0, 0,
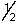

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
77
The outermost or highest energy electron in element 105 could be characterized by which of the following sets of n, l, ml and ms quantum numbers?
A) 7, 3, -3, -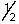
B) 6, 3, -1, -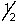
C) 6, 2, 0,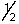
D) 5, 3, -1, -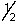
E) 5, 2, 0,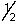
A) 7, 3, -3, -
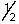
B) 6, 3, -1, -
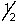
C) 6, 2, 0,
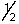
D) 5, 3, -1, -
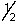
E) 5, 2, 0,

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following describes a possible set of quantum numbers for the last electron added to form an aluminum atom when atomic orbitals are filled?
A) 1, 0, 0, +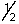
B) 2, 0, 0, +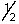
C) 2, l, 1, +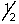
D) 3, 0, 0, +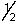
E) 3, 1, 1, +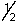
A) 1, 0, 0, +
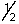
B) 2, 0, 0, +
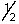
C) 2, l, 1, +
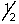
D) 3, 0, 0, +
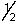
E) 3, 1, 1, +

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the lowest energy electronic configuration of a fluorine atom?
A) 1s2 2s2 2p5
B) 1s2 2s2 2p6
C) 1s2 2s2 2p7
D) 1s2 2s2 2p6 3s1
E) none of the above
A) 1s2 2s2 2p5
B) 1s2 2s2 2p6
C) 1s2 2s2 2p7
D) 1s2 2s2 2p6 3s1
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
80
What is the value of x in the following electron configuration for silicon?
1s2 2s2 2p6 3s2 3px
A) 1
B) 2
C) 3
D) 4
E) 6
1s2 2s2 2p6 3s2 3px
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck








