Deck 12: Gas Laws
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/78
العب
ملء الشاشة (f)
Deck 12: Gas Laws
1
Indicate the missing words in the following statement: "Gas laws describe in mathematical terms the relationships between the variables pressure, ________ and ________ for a fixed quantity of gas."
A) temperature; chemical identity
B) temperature; volume
C) volume; chemical identity
D) chemical identity; cost
A) temperature; chemical identity
B) temperature; volume
C) volume; chemical identity
D) chemical identity; cost
temperature; volume
2
Which of the following is/are characteristics of gases?
A) high compressibility
B) relatively long distances between molecules
C) formation of homogeneous mixtures regardless of the natures of non-reacting gas components
D) all of the above
A) high compressibility
B) relatively long distances between molecules
C) formation of homogeneous mixtures regardless of the natures of non-reacting gas components
D) all of the above
all of the above
3
Which of the following is not a unit used in measuring pressure?
A) atmosphere
B) inches Hg
C) kilometer Hg
D) millimeters Hg
A) atmosphere
B) inches Hg
C) kilometer Hg
D) millimeters Hg
kilometer Hg
4
In terms of size, the mm Hg pressure unit is ________ the torr unit.
A) 100 times larger than
B) numerically equal to
C) 760 times larger than
D) 1000 times larger than
A) 100 times larger than
B) numerically equal to
C) 760 times larger than
D) 1000 times larger than

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is standard atmospheric pressure in inches mercury (in Hg)? (2.54 cm = 1 in)
A) 1930 in Hg
B) 101 in Hg
C) 76.0 in Hg
D) 29.9 in Hg
A) 1930 in Hg
B) 101 in Hg
C) 76.0 in Hg
D) 29.9 in Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
6
The barometric pressure in the eye of a hurricane sometimes dips as low as 27.2 inches mercury. How many millimeters of mercury is this?
A) 1.1 mm Hg
B) 6.9 mm Hg
C) 691 mm Hg
D) 107 mm Hg
A) 1.1 mm Hg
B) 6.9 mm Hg
C) 691 mm Hg
D) 107 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
7
8.00 liters of oxygen gas is under a pressure of 280 torr. If the volume of this gas is increased to 14.00 liters at constant temperature, what will the new pressure be?
A) 66 torr
B) 490 torr
C) 387 torr
D) 160 torr
A) 66 torr
B) 490 torr
C) 387 torr
D) 160 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
8
Indicate what the missing words are in the following statement of Boyle's law: At constant temperature, the volume of a gas sample is ________ proportional to its ________.
A) directly, gas constant
B) directly, pressure
C) inversely, mass
D) inversely, pressure
A) directly, gas constant
B) directly, pressure
C) inversely, mass
D) inversely, pressure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
9
A 2.50 L sample of helium gas has a pressure of 0.925 atm. Calculate the pressure of the gas if the volume is reduced to 0.350 L at constant temperature.
A) 0.130 atm
B) 0.946 atm
C) 6.61 atm
D) 0.661 atm
A) 0.130 atm
B) 0.946 atm
C) 6.61 atm
D) 0.661 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
10
A mathematical statement of Charles' law is ________.
A) V1T1 = V2T2
B) V1 + T1 = V2 + T2
C)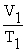 =
= 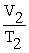
D)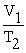 =
= 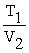
A) V1T1 = V2T2
B) V1 + T1 = V2 + T2
C)
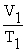 =
= 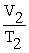
D)
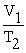 =
= 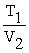

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following statements best gives the kinetic molecular theory explanation of the Boyle's law observation of increased pressure due to decreased volume?
A) Particles have more kinetic energy.
B) Particles increase in size.
C) Particles strike container walls with more force.
D) Particles strike container walls more frequently.
A) Particles have more kinetic energy.
B) Particles increase in size.
C) Particles strike container walls with more force.
D) Particles strike container walls more frequently.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
12
Charles' law involves which of the following?
A) a varying temperature
B) a constant volume
C) a varying mass of gas
D) an indirect proportion
A) a varying temperature
B) a constant volume
C) a varying mass of gas
D) an indirect proportion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
13
10.0 liters of oxygen gas is at a temperature of 23 °C. If the temperature of the gas is raised to 40 °C at constant pressure the new volume will be ________.
A) 1.8 liters
B) 4.2 liters
C) 14.1 liters
D) 10.6 liters
A) 1.8 liters
B) 4.2 liters
C) 14.1 liters
D) 10.6 liters

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the final Celsius temperature if 6.48 L of H2 gas at 22.0 °C is cooled until the volume reaches 3.77 L?
A) 24.8 °C
B) 113 °C
C) -205 °C
D) -101 °C
A) 24.8 °C
B) 113 °C
C) -205 °C
D) -101 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
15
If the volume of a sample of chlorine gas is reduced by 1/3 at constant pressure, what happens to its absolute temperature?
A) It remains constant.
B) It decreases by 1/3.
C) It triples.
D) It increases nine-fold.
A) It remains constant.
B) It decreases by 1/3.
C) It triples.
D) It increases nine-fold.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
16
The Gay-Lussac's law observation of increased pressure due to increased temperature would be explained using kinetic theory in the following way: The pressure must increase because ________.
A) the molecules will strike the container walls less often
B) the molecules will strike the container walls more often
C) the molecules can move slower
D) the molecules can increase in size
A) the molecules will strike the container walls less often
B) the molecules will strike the container walls more often
C) the molecules can move slower
D) the molecules can increase in size

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
17
A mathematical statement of Gay-Lussac's law is ________.
A) P1 + T2 = P2 + T2
B) P1T1 = P2T2
C)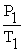 =
= 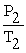
D)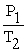 =
= 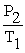
A) P1 + T2 = P2 + T2
B) P1T1 = P2T2
C)
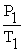 =
= 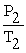
D)
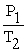 =
= 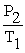

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
18
According to Gay-Lussac's law, if the pressure of a gas sample is doubled the gas sample ________.
A) volume decreases by a factor of 2
B) volume is doubled
C) Kelvin temperature decreases by a factor of 2
D) Kelvin temperature is doubled
A) volume decreases by a factor of 2
B) volume is doubled
C) Kelvin temperature decreases by a factor of 2
D) Kelvin temperature is doubled

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
19
How would the pressure of a sealed container of gas be affected if its Kelvin temperature is halved while the moles and the volume of the gas remain constant?
A) The pressure would be reduced to one half of the original pressure.
B) The pressure would quadruple.
C) The pressure would not be affected.
D) The pressure would be reduced by a factor of 4.
A) The pressure would be reduced to one half of the original pressure.
B) The pressure would quadruple.
C) The pressure would not be affected.
D) The pressure would be reduced by a factor of 4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
20
A cylinder of oxygen gas has a pressure of 6.00 atm at 25.0 °C. What is the pressure of the gas at 0.0 °C?
A) 5.50 atm
B) 6.55 atm
C) 2.40 atm
D) 15.0 atm
A) 5.50 atm
B) 6.55 atm
C) 2.40 atm
D) 15.0 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
21
According to the combined gas law, for a fixed amount of gas ________.
A) volume and pressure are directly proportional
B) volume and Kelvin temperature are inversely proportional
C) Kelvin and pressure are directly proportional
D) pressure and Kelvin temperature have no relationship to each other
A) volume and pressure are directly proportional
B) volume and Kelvin temperature are inversely proportional
C) Kelvin and pressure are directly proportional
D) pressure and Kelvin temperature have no relationship to each other

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
22
The combined gas law cannot be written as ________.
A)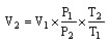
B)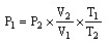
C)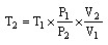
D)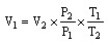
A)
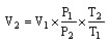
B)
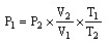
C)
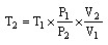
D)
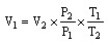

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
23
If both the pressure and the Kelvin temperature of a gas are halved, the volume will ________.
A) double
B) quadruple
C) be halved
D) remain the same
A) double
B) quadruple
C) be halved
D) remain the same

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
24
How will the pressure of a fixed sample of gas change if its volume is halved and its temperature is quadrupled (moles of gas remain constant)?
A) The pressure will increase by a factor of 4.
B) The pressure will increase by a factor of 8
C) The pressure will quadruple.
D) The pressure will decrease by a factor of 8.
A) The pressure will increase by a factor of 4.
B) The pressure will increase by a factor of 8
C) The pressure will quadruple.
D) The pressure will decrease by a factor of 8.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
25
Syringe A has a specified volume of a gas at room temperature and atmospheric pressure. Which response below would not change the volume of gas in syringe A to the volume of gas shown in syringe B?

A) Decrease the pressure of the gas in syringe A at constant temperature.
B) Increase the temperature of the gas in syringe A at constant pressure.
C) Increase the pressure of the gas in syringe A at constant temperature.
D) Decrease the pressure, and increase the temperature on the gas in syringe A.

A) Decrease the pressure of the gas in syringe A at constant temperature.
B) Increase the temperature of the gas in syringe A at constant pressure.
C) Increase the pressure of the gas in syringe A at constant temperature.
D) Decrease the pressure, and increase the temperature on the gas in syringe A.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
26
A sample of nitrogen gas occupies a volume of 8.65 L at a temperature of 295 K and a pressure of 0.985 atm. What is the pressure of the gas if the volume of the gas is increased to 12.1 L and the temperature is reduced to 284 K?
A) 1.08 atm
B) 0.875 atm
C) 0.678 atm
D) 1.39 atm
A) 1.08 atm
B) 0.875 atm
C) 0.678 atm
D) 1.39 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
27
The conditions known as STP are ________.
A) 760 atm and 273 K
B) 760 mm Hg and 273 °C
C) 1 atm and 273 K
D) 1 mm Hg and 273 K
A) 760 atm and 273 K
B) 760 mm Hg and 273 °C
C) 1 atm and 273 K
D) 1 mm Hg and 273 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
28
A sample of N2 gas occupies a volume 180 mL at STP. What volume, in L, will it occupy at 640 mm Hg and 295 K?
A) 0.231 L
B) 1.45 L
C) 1.07 L
D) 0.690 L
A) 0.231 L
B) 1.45 L
C) 1.07 L
D) 0.690 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
29
Gay-Lussac's law of combining volumes states that the volumes of gases involved in a chemical reaction are in the same ratio as the coefficients for these gases in the balanced equation for the reaction provided that ________.
A) all volumes are measured in liters
B) all volumes are measured at the same temperature and pressure
C) all the gases have molecules that contain the same number of atoms
D) all the gases form diatomic molecules
A) all volumes are measured in liters
B) all volumes are measured at the same temperature and pressure
C) all the gases have molecules that contain the same number of atoms
D) all the gases form diatomic molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
30
What volume of H2 gas at 780 mm Hg and 23 °C is required to produce 10.6 L of NH3 gas at the same temperature and pressure using the following chemical reaction?
N2(g) + 3H2(g) 2NH3(g)
A) 15.9 L
B) 13.0 L
C) 20.0 L
D) 15.0 L
N2(g) + 3H2(g) 2NH3(g)
A) 15.9 L
B) 13.0 L
C) 20.0 L
D) 15.0 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
31
Propane burners are used by campers for cooking purposes while in the wild. What volume of H2O gas is produced by the complete combustion of 1.8 L of propane (C3H8) gas? All gas volumes are measured at the same temperature and pressure.
C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g)
A) 1.8 L
B) 14 L
C) 7.2 L
D) 0.52 L
C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g)
A) 1.8 L
B) 14 L
C) 7.2 L
D) 0.52 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
32
At STP the volume of HCl that will result from the reaction of 0.680 L of H2 and 0.750 L of Cl2 is ________.
H2(g) + Cl2(g) 2 HCl(g)
A) 0.680 L
B) 0.750 L
C) 1.36 L
D) 0.340 L
H2(g) + Cl2(g) 2 HCl(g)
A) 0.680 L
B) 0.750 L
C) 1.36 L
D) 0.340 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
33
Commercial ammonia is produced by reacting hydrogen gas with nitrogen gas according to the following equation.
N2(g) + 3 H2(g) 2 NH3(g)
If 6.00 × 104 L of N2 are reacted with 1.2 × 105 L of H2, the limiting reactant is ________ and the volume of ammonia produced is ________ L.
A) N2, 3.33 x 103
B) H2, 6.00 x 103
C) N2, 8.00 x 104
D) H2, 8.00 x 104
N2(g) + 3 H2(g) 2 NH3(g)
If 6.00 × 104 L of N2 are reacted with 1.2 × 105 L of H2, the limiting reactant is ________ and the volume of ammonia produced is ________ L.
A) N2, 3.33 x 103
B) H2, 6.00 x 103
C) N2, 8.00 x 104
D) H2, 8.00 x 104

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following two variables are present in mathematical statements of Avogadro's law?
A) n and V
B) n and T
C) n and P
D) n, P, and V
A) n and V
B) n and T
C) n and P
D) n, P, and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
35
The molar volume of a gas is the volume occupied at STP by ________.
A) 22,400 mL of gas
B) 760 L of gas
C) 22.41 g of gas
D) 6.02 x 1023 g of gas
A) 22,400 mL of gas
B) 760 L of gas
C) 22.41 g of gas
D) 6.02 x 1023 g of gas

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
36
At standard conditions, one mole of any gas will occupy a volume of ________.
A) 1.00 liter
B) 22.41 mL
C) 22.41 L
D) 22,400 L
A) 1.00 liter
B) 22.41 mL
C) 22.41 L
D) 22,400 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the volume at STP, in liters, occupied by 26.4 g of F2 gas?
A) 4.28 L
B) 15.6 L
C) 12.4 L
D) 11.8 L
A) 4.28 L
B) 15.6 L
C) 12.4 L
D) 11.8 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
38
Three 1.0 L flasks filled with H2, O2 and He, respectively, are at STP. Which of the following statements concerning these gases is true?
A) Each flask contains the same number of atoms.
B) There are twice as many H2 and O2 molecules as there are He atoms.
C) There are twice as many He atoms as there are H2 or O2 molecules.
D) The number of H2 or O2 molecules is the same as the number of He atoms.
A) Each flask contains the same number of atoms.
B) There are twice as many H2 and O2 molecules as there are He atoms.
C) There are twice as many He atoms as there are H2 or O2 molecules.
D) The number of H2 or O2 molecules is the same as the number of He atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
39
What volume is occupied by 6.21 x 1024 molecules of CO at STP?
A) 106 L
B) 230 L
C) 44.3 L
D) 231 L
A) 106 L
B) 230 L
C) 44.3 L
D) 231 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
40
A 15.0 L cylinder was filled with of O2 gas at STP. Calculate the number of O2 molecules in the cylinder.
A) 4.03 x 1023 molecules
B) 2.77 x 1022 molecules
C) 443 molecules
D) 6.59 x 1024 molecules
A) 4.03 x 1023 molecules
B) 2.77 x 1022 molecules
C) 443 molecules
D) 6.59 x 1024 molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
41
Calculate the density of butane (C4H10) in grams per liter at STP conditions.
A) 0.822 g/L
B) 2.60 g/L
C) 3.79 g/L
D) 5.48 g/L
A) 0.822 g/L
B) 2.60 g/L
C) 3.79 g/L
D) 5.48 g/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
42
Calculate the density of ammonia (NH3) gas in a 4.32 L container at 837 torr and 45 °C.
A) 3.86 g/L
B) 0.719 g/L
C) 0.432 g/L
D) 0.194 g/L
A) 3.86 g/L
B) 0.719 g/L
C) 0.432 g/L
D) 0.194 g/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
43
The density of a gaseous chlorofluorocarbon (CFC) at 23.8 °C and 432 mmHg is 3.23 g/L. What is its molar mass?
A) 0.182 g/mol
B) 139 g/mol
C) 11.1 g/mol
D) 0.0146 g/mol
A) 0.182 g/mol
B) 139 g/mol
C) 11.1 g/mol
D) 0.0146 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
44
6.80 L of CH4 gas at 0.980 atm and 23 °C ________.
A) contains 0.897 mol CH4
B) has a mass of 3.13 g
C) contains 6.02 x 1022 molecules CH4
D) contains 6.61 x 1023 H atoms
A) contains 0.897 mol CH4
B) has a mass of 3.13 g
C) contains 6.02 x 1022 molecules CH4
D) contains 6.61 x 1023 H atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
45
The ideal gas equation cannot be written as ________.
A) PV = nRT
B) P =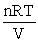
C) R =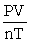
D) R =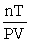
A) PV = nRT
B) P =
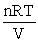
C) R =
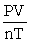
D) R =
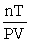

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
46
The value of the ideal gas constant, for the units (torr mL)/mole K is ________.
A) 0.0821
B) 1/0.0821
C) 62.4
D) 62,400
A) 0.0821
B) 1/0.0821
C) 62.4
D) 62,400

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
47
The temperature, in °C, occupied by 0.643 moles of O2 gas at 0.893 atm pressure and a volume of 14.7 L is ________.
A) 16 °C
B) -24 °C
C) 48 °C
D) 318 °C
A) 16 °C
B) -24 °C
C) 48 °C
D) 318 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
48
At what temperature in °C does 0.444 mole of carbon monoxide, CO, occupy 11.8 L at 889 mmHg?
A) 51 °C
B) 14 °C
C) 32 °C
D) 106 °C
A) 51 °C
B) 14 °C
C) 32 °C
D) 106 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
49
Calculate the volume of 12.0 g of helium at 54.5 °C and 1025 mm Hg.
A) 59.8 L
B) 13.9 L
C) 24.6 L
D) 77.4 L
A) 59.8 L
B) 13.9 L
C) 24.6 L
D) 77.4 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following is a correct expression for the molecular mass of a gas?
A)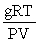
B)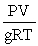
C)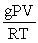
D)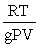
A)
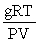
B)
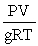
C)
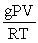
D)
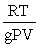

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
51
Of the following gases, the one with the greatest density at STP is ________.
A) NO
B) N2
C) O2
D) CO2
A) NO
B) N2
C) O2
D) CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
52
The density of a gas is 1.34 g/L at STP. What is the mass of one mole of this gas?
A) 30.0 g
B) 28.0 g
C) 44.0 g
D) 43.9 g
A) 30.0 g
B) 28.0 g
C) 44.0 g
D) 43.9 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the density of F2, if the temperature is 24.0 °C and the pressure is 0.986 atm?
A) 1.24 g/L
B) 1.54 g/L
C) 2.04 g/L
D) 1.96 g/L
A) 1.24 g/L
B) 1.54 g/L
C) 2.04 g/L
D) 1.96 g/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which noble gas has a density of 5.86 g/L at STP?
A) Ne
B) Ar
C) Kr
D) Xe
A) Ne
B) Ar
C) Kr
D) Xe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
55
A 0.50 L cylinder contains 0.820 grams of an unknown gas. The pressure inside the cylinder is 2.50 atmospheres at 25.0 °C. What is the identity of the unknown gas?
A) 28.0 g/mol, N2
B) 2.02 g/mol, H2
C) 16.0 g/mol, CH4
D) 38.0 g/mol, F2
A) 28.0 g/mol, N2
B) 2.02 g/mol, H2
C) 16.0 g/mol, CH4
D) 38.0 g/mol, F2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
56
0.2050 g of a liquid was added to an Erlenmeyer flask. The temperature inside the flask was 35.°C. The volume of the flask was 125 mL. The molecular weight of the liquid was 44.0 g/mole. Calculate the pressure inside the flask in mm Hg.
A) 460 mm Hg
B) 58.1 mm Hg
C) 717 mm Hg
D) 1.40 x 103 mm Hg
A) 460 mm Hg
B) 58.1 mm Hg
C) 717 mm Hg
D) 1.40 x 103 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
57
At 150 °C and 1.00 atm, 500 mL of a vapor has a mass of 0.8365 g. What is the molecular weight of the compound?
A) 110 g/mol
B) 116 g/mol
C) 130 g/mol
D) 58.1 g/mol
A) 110 g/mol
B) 116 g/mol
C) 130 g/mol
D) 58.1 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
58
In the reaction Fe2O3 + 3H2 2Fe + 3H2O, how many grams of Fe2O3 must react to produce 10.8 liters of H2O at STP?
A) 25.7 g
B) 12.6 g
C) 65.3 g
D) 648 g
A) 25.7 g
B) 12.6 g
C) 65.3 g
D) 648 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
59
How many grams of sodium azide, NaN3 (molar mass = 65.02) are needed to react by the equation below in order to inflate a 66.0 L air bag with N2(g) at 294 K and 0.989 atm pressure?
2 NaN3(s) 2 Na(s) + 3 N2(g)
A) 44.2 g
B) 60.1 g
C) 95.1 g
D) 117 g
2 NaN3(s) 2 Na(s) + 3 N2(g)
A) 44.2 g
B) 60.1 g
C) 95.1 g
D) 117 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
60
Electrolysis of 12.6 g of water would produce what volume of O2 gas at 640 torr and
22)0 °C?
2 H2O (l) + electricity 2 H2(g) + O2(g)
A) 10.1 L
B) 44.8 L
C) 22.4 L
D) 68.4 L
22)0 °C?
2 H2O (l) + electricity 2 H2(g) + O2(g)
A) 10.1 L
B) 44.8 L
C) 22.4 L
D) 68.4 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
61
What volume of H2, in milliliters, at 21 °C and 0.998 atm can be produced from the reaction of 2.40 grams of Zn in excess HCl?
Zn(s) + 2 HCl(aq) ZnCl2(aq) + H2(g)
A) 890 mL
B) 675 mL
C) 3300 mL
D) 1200 mL
Zn(s) + 2 HCl(aq) ZnCl2(aq) + H2(g)
A) 890 mL
B) 675 mL
C) 3300 mL
D) 1200 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
62
The pressure exerted by a gas collected by water displacement is obtained by subtracting the vapor pressure of H2O from the total pressure of the gas collected. This is an example of the application of ________.
A) Avogadro's law
B) Dalton's law
C) Boyle's law
D) Charles' law
A) Avogadro's law
B) Dalton's law
C) Boyle's law
D) Charles' law

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the volume occupied by a mixture of 25.7 g of NO2 and 13.9 g of SO2 at STP?
A) 11.2 L
B) 22.4 L
C) 17.4 L
D) 26.9 L
A) 11.2 L
B) 22.4 L
C) 17.4 L
D) 26.9 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
64
A mixture of 8.95 g of Cl2 and 18.0 g of NO2 and an undetermined amount of SO2 occupies a volume of 22.4 liters at 760 mm Hg and 0 °C. How many moles of SO2 are present?
A) 1.06 moles
B) 2.77 moles
C) 0.381 moles
D) 0.483 moles
A) 1.06 moles
B) 2.77 moles
C) 0.381 moles
D) 0.483 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the partial pressure of N2 in a mixture of 0.36 mole N2, 0.15 mole Ne, and 0.75 mole of O2 that has a total pressure of 3.18 atm?
A) 0.29 atm
B) 0.36 atm
C) 0.65 atm
D) 0.91 atm
A) 0.29 atm
B) 0.36 atm
C) 0.65 atm
D) 0.91 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the total pressure in a 4.00 L flask at 298 K, that contains 2.6 moles of He, 0.8 mole of Ne, 1.6 moles of O2?
A) 2.6 atm
B) 4.9 atm
C) 31 atm
D) 25 atm
A) 2.6 atm
B) 4.9 atm
C) 31 atm
D) 25 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the volume percent of Ar in a 6.50 L flask that contains 0.200 mole of Ne, 0.300
Mole He, 0.600 mole of Ar at STP?
A) 36.4%
B) 54.5%
C) 44.3%
D) 21.9%
Mole He, 0.600 mole of Ar at STP?
A) 36.4%
B) 54.5%
C) 44.3%
D) 21.9%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
68
An excimer laser contains 0.40 mole Ar and 0.60 mole F2. The total pressure inside the laser cavity is 1.10 atm. What is the partial pressure of Ar inside the laser?
A) 0.40 atm
B) 0.44 atm
C) 0.60 atm
D) 1.00 atm
A) 0.40 atm
B) 0.44 atm
C) 0.60 atm
D) 1.00 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
69
A sample of SO3 gas is decomposed to SO2 and O2.
2 SO3(g) 2 SO2(g) + O2(g)
If the total pressure of SO2 and O2 is 1340 torr, calculate the partial pressure of O2 in torr.
A) 1340 torr
B) 893 torr
C) 447 torr
D) 1160 torr
2 SO3(g) 2 SO2(g) + O2(g)
If the total pressure of SO2 and O2 is 1340 torr, calculate the partial pressure of O2 in torr.
A) 1340 torr
B) 893 torr
C) 447 torr
D) 1160 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
70
A gas has a volume of 225 mL at a pressure of 162.5 torr. What is the pressure (in atm) if the volume is decreased to 45 mL?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
71
A quantity of gas has a volume of 3560 mL at a temperature of 55 °C and a pressure of 850 torr. What is the temperature (in °C) if the volume remains unchanged but the pressure is decreased to 0.652 atm?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
72
Chlorine gas is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 is 6.18 L at 0.884 atm and 46 °C, at what temperature (in °C) will the volume be 2.00 L if the pressure is 157 kPa?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
73
A sample of helium gas has a volume of 1.25 L at -125 °C and 5.00 atm. This gas is compressed at 50.0 atm to a volume of 325 mL. What is the final temperature of the helium gas in °C?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the pressure, exerted by 1.00 x 1020 molecules of nitrogen in a 305 mL flask at
175 °C?
175 °C?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
75
An unknown gas with a mass of 4.52 g was found to occupy a volume of 875 mL at 1.69 atm and 65 °C. What is the molecular weight of this gas?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
76
Monochloroethylene is used to make polyvinylchloride (PVC). It has a density of 2.56 g/L at 22.8 °C and 756 mmHg. What is the molar mass of monochloroethylene?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
77
What mass of NH3 gas has a volume of 16,400 mL, a pressure of 0.955 atm and a temperature of -23 °C?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck
78
A mixture of 8.50 g of hydrogen and 15.0 g of helium in a 7.3 L flask is maintained at 15.0 °C. What is the total pressure in the container?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 78 في هذه المجموعة.
فتح الحزمة
k this deck








