Deck 10: Sublimation
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/4
العب
ملء الشاشة (f)
Deck 10: Sublimation
1
Which of the following compounds could be subjected to sublimation at atmospheric pres- sure?
a) Compound A: vapor pressure at its melting point = 770 mm Hg
(b) Compound B: vapor pressure at its melting point = 400 mm Hg
(c) Compound C: vapor pressure at its melting point = 10 mm Hg
a) Compound A: vapor pressure at its melting point = 770 mm Hg
(b) Compound B: vapor pressure at its melting point = 400 mm Hg
(c) Compound C: vapor pressure at its melting point = 10 mm Hg
Compounds A and B will have significant vapor pressure at their melting point.
2
In the preceding problem, which compounds could be vacuum sublimated?
Only compund C. The others would have such significant vapor pressure in a vacuum that they would be lost into the vacuum system.
3
3 Which of the following compounds would be likely to evaporate readily? Explain your answer.
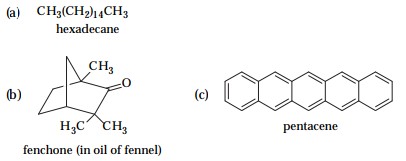

Only (b) and (c). Fenchone will evaporate readily because it is spherically shaped. Pentacene will evaporate becaue it is cylindricaly shaped. Hexadecane has a zig-zag shape and strong van der Waal attractions.
4
You have a mixture of p-nitrobenzaldehyde (vapor pressure 0.01 at its melting point of 106˚) and camphor. Explain how you would separate these two compounds by sublimation. How would you perform the sublimation if benzoic acid instead of camphor is used?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 4 في هذه المجموعة.
فتح الحزمة
k this deck








