Deck 17: Proton Nuclear Magnetic Resonance Spectroscopy
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/8
العب
ملء الشاشة (f)
Deck 17: Proton Nuclear Magnetic Resonance Spectroscopy
1
List the following protons in order of increasing chemical shift (smallest shift first).
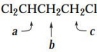
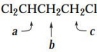
b > c > a, because of inductive effects.
2
Pyrrole shows two principal CH signals in its NMR spectrum: at 6.5 ppm and 6.7 ppm. Is pyr- role an aromatic compound or a conjugated diene?
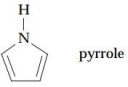 Absorption is downfield in the aromatic region; therefore, pyrrole is an aromatic compound.
Absorption is downfield in the aromatic region; therefore, pyrrole is an aromatic compound. 3
How many types of nonequivalent protons does each of the following compounds contain? (Example: CH3CH2Cl has two types, CH3 and CH2.)


(a) two
(b) three
(c) three
(d) two
(e) one
(b) three
(c) three
(d) two
(e) one
4
In the preceding problem, determine the relative areas under the principal signals for each compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 8 في هذه المجموعة.
فتح الحزمة
k this deck
5
5 What would be the splitting pattern (singlet, doublet, etc.) observed for each of the following indicated protons?
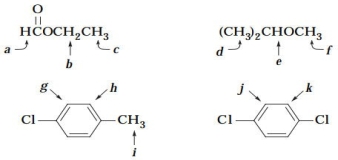


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 8 في هذه المجموعة.
فتح الحزمة
k this deck
6
A NMR spectrum shows a singlet at 7.3 ppm, a triplet at 4.3, a triplet at 2.9, and a singlet at 2.0. The relative areas are 5, 2, 2, and 3, respectively. Which of the following compounds is compat- ible with this spectrum?



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 8 في هذه المجموعة.
فتح الحزمة
k this deck
7
How would you distinguish between the following pairs of compounds using NMR spectros- copy? (Include in your answer the expected splitting patterns and relative areas of the NMR absorption.)



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 8 في هذه المجموعة.
فتح الحزمة
k this deck
8
The following types of protons (underlined) all show NMR absorption between d = 10-16 ppm. How would you use the infrared spectrum to identify the type of compound?



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 8 في هذه المجموعة.
فتح الحزمة
k this deck








