Deck 27: Synthetic Polymers
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/12
العب
ملء الشاشة (f)
Deck 27: Synthetic Polymers
1
Which of the following is the monomer that gives the polymer shown below? 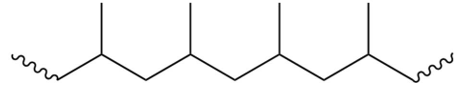
A)
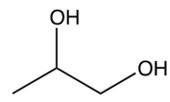
B)
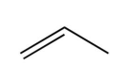
C)
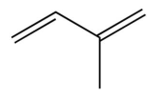
D)
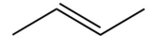

A)

B)

C)

D)


2
Which one of the following monomers undergoes cationic polymerization most readily?
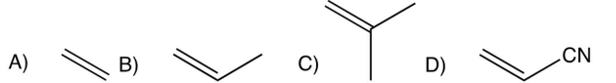
A)
B)
C)
D)

A)
B)
C)
D)
3
Which one of the following monomers undergoes anionic polymerization most readily?
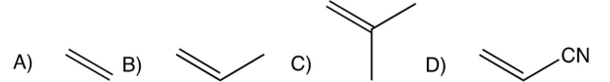
A)
B)
C)
D)

A)
B)
C)
D)
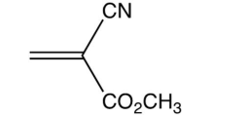
4
The monomer used to make superglue is shown below. Which one of the following methods of polymerization is most suitable for this type of monomer?
A) free-radical chain-growth
B) cationic chain-growth
C) anionic chain-growth
D) acid-catalyzed step-growth

A) free-radical chain-growth
B) cationic chain-growth
C) anionic chain-growth
D) acid-catalyzed step-growth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
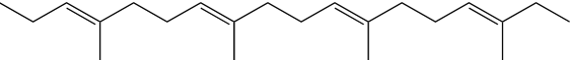
5
Which one of the following is the monomer that gives the polymer shown below?
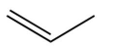
A)
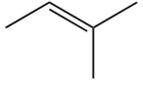
B)
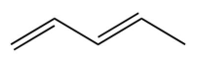
C)
D)
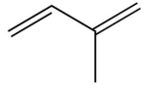

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
6
Identify the repeating unit in the polymer formed from the following reaction sequence. 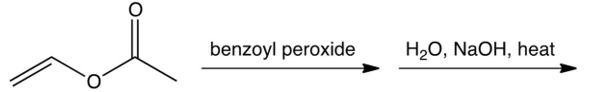
A)
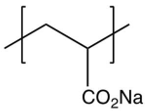
B)
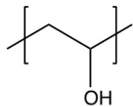
C)
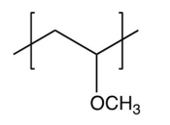
D)
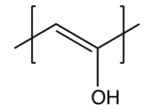
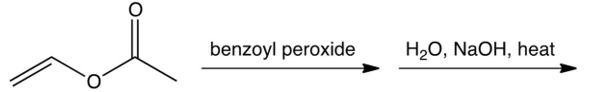
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
7
The acid-catalyzed dimerization of isobutylene gives a mixture of two isomeric alkenes (A and ). Hydrogenation of this mixture gives a single hydrocarbon. What is the hydrocarbon?

A) 2,2,4-trimethylpentane
B) 2,3,4-trimethylpentane
C) 2,4-dimethylhexane
D) 2,5-dimethyhexane

A) 2,2,4-trimethylpentane
B) 2,3,4-trimethylpentane
C) 2,4-dimethylhexane
D) 2,5-dimethyhexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
8
The repeating unit of poly (methyl methacrylate) is shown below. Which one of the following is the monomer used to make poly (methyl methacrylate)?
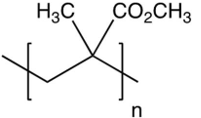
A)
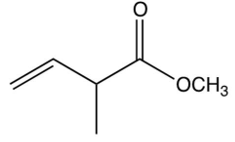
B)
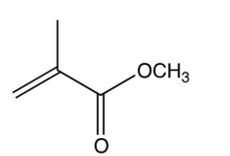
C)
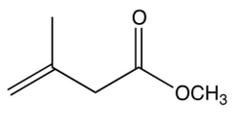
D)
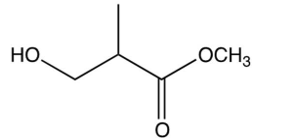

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following monomers is used to make the polymer carbowax, shown below? 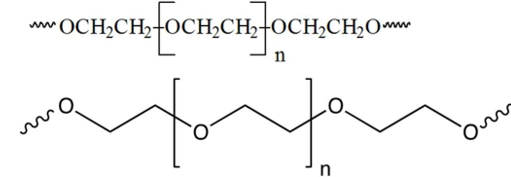
A)
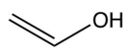
B)
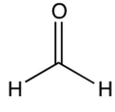
C)
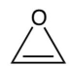
D)
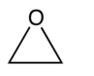

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which one of the following initiators can be used for anionic chain-growth polymerization?
A) benzoyl peroxide
B)
C)
D)
A) benzoyl peroxide
B)
C)
D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which one of the following initiators can be used for free radical chain-growth polymerization?
A) benzoyl peroxide
B)
C)
D)
A) benzoyl peroxide
B)
C)
D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which one of the following initiators is used to make isotactic polypropylene?
A) benzoyl peroxide
B)
C)
D)
A) benzoyl peroxide
B)
C)
D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 12 في هذه المجموعة.
فتح الحزمة
k this deck








