Deck 1: The Basics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/14
العب
ملء الشاشة (f)
Deck 1: The Basics
1
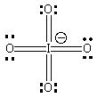
Which of the following is the best Lewis Structure for the IO4- ion? 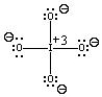
A)
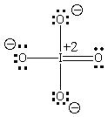
B)
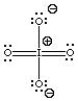
C)
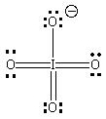
D)

A)

B)

C)

D)


2
What is the formal charge of Nitrogen in the following structure? 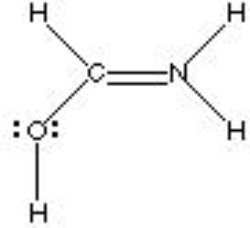
A) +2
B) +1
C) 0
D) -1
E) -2

A) +2
B) +1
C) 0
D) -1
E) -2
+1
3
What is the formal charge of Oxygen in the following structure? 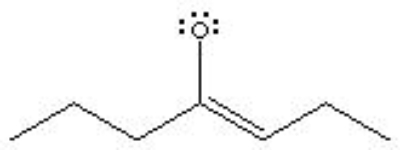
A) +2
B) +1
C) 0
D) -1
E) -2

A) +2
B) +1
C) 0
D) -1
E) -2
-1
4
Which of the following are correct resonance structures of structure I? 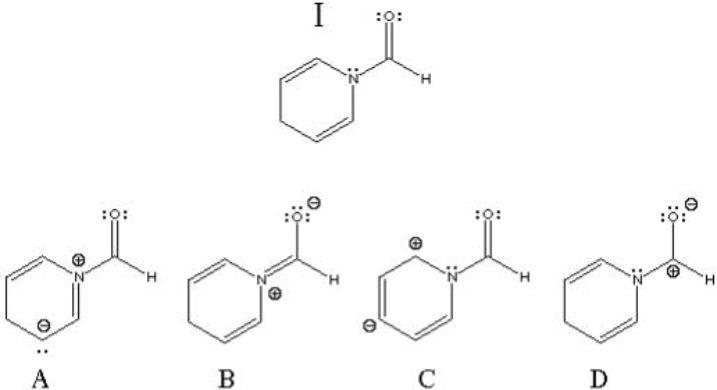
A) A and B
B) B, C and D
C) A, B and D
D) all of them
E) none of them

A) A and B
B) B, C and D
C) A, B and D
D) all of them
E) none of them

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the best resonance structure for structure I? 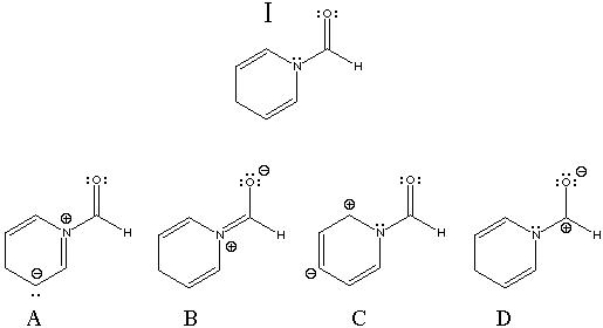
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the hybridization of the indicated atom in the following structure?
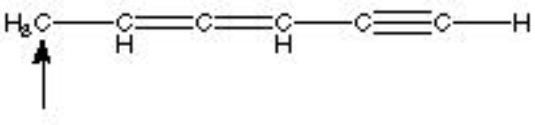
A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the hybridization of the indicated atom in the following structure?
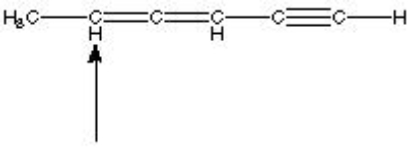
A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the correct molecular orbital diagram for the following structure
?
A)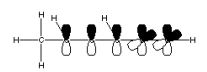
B)
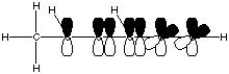
C)
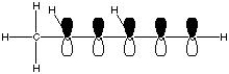
D)
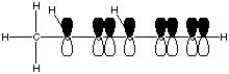
E)
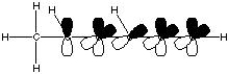
?
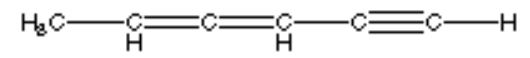
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the hybridization of the indicated atom in the following structure? 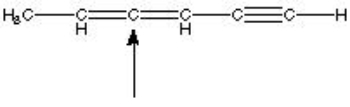
A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the hybridization of the indicated atom in the following structure? 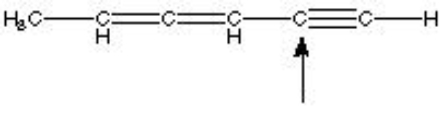
A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

A) sp3
B) sp2
C) sp
D) sp2d2
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
11
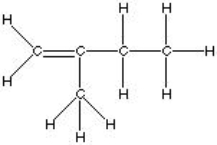
Which of the following is the best, correct Lewis Structure for CH2C(CH3)CH2CH3?
A)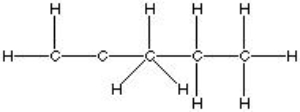
B)
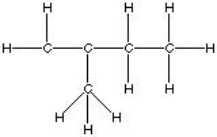
C)
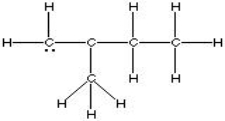
D)
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the choices is equivalent to CH3CH2CH(OH)CH2CH3?
A)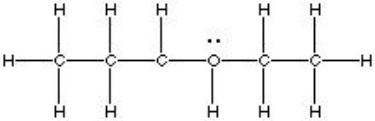
B) C5H11O
C)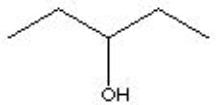
D)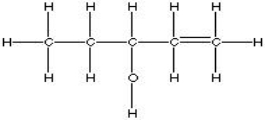
A)

B) C5H11O
C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which is the correct bond-line formula for the following structure?
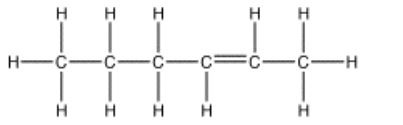
A)
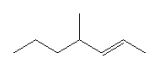
B)

C)
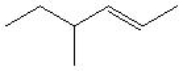
D)
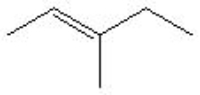

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which is the correct condensed formula for the following structure? 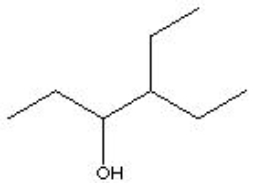
A) CH3CH2CH(OH)CHCH2CH3
B) CH3CH2CH(OH)CH(CH2CH3)2
C) CH3CH2(OH)CH(CH2CH3)CH3
D) CH3CH2CH2(OH)CH(CH2CH3)CH2CH3

A) CH3CH2CH(OH)CHCH2CH3
B) CH3CH2CH(OH)CH(CH2CH3)2
C) CH3CH2(OH)CH(CH2CH3)CH3
D) CH3CH2CH2(OH)CH(CH2CH3)CH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 14 في هذه المجموعة.
فتح الحزمة
k this deck








