Deck 2: Acids and Bases Central to Understanding Organic Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/10
العب
ملء الشاشة (f)
Deck 2: Acids and Bases Central to Understanding Organic Chemistry
1
Which compound is the conjugate base of CH3OH?
A) CH3OH2+
B) CH3O-
C)-CH2OH
D) HO-
E) H2O
A) CH3OH2+
B) CH3O-
C)-CH2OH
D) HO-
E) H2O
CH3O-
2
Which of the following is the most acidic?
Approximate pKa
A) CH3OH
B) CH3OH2+
C) CH3NH2
D) CH3NH3+
E) CH3CO2H
Approximate pKa
A) CH3OH
B) CH3OH2+
C) CH3NH2
D) CH3NH3+
E) CH3CO2H
CH3OH2+
3
Which of the following acid-base reactions favor the products?
A) HF + H2O ? F- + H3O+
B)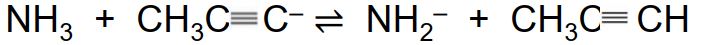
C) CH3CH3 + H2C=CH- ? CH3CH2- + H2C=CH2
D) CH3CO2H + CH3CH2O- ? CH3CO2- + CH3CH2OH
E) CH3OH + CH3CO2H ? CH3OH2+ + CH3CO2-
A) HF + H2O ? F- + H3O+
B)
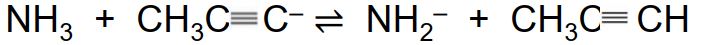
C) CH3CH3 + H2C=CH- ? CH3CH2- + H2C=CH2
D) CH3CO2H + CH3CH2O- ? CH3CO2- + CH3CH2OH
E) CH3OH + CH3CO2H ? CH3OH2+ + CH3CO2-
CH3CO2H + CH3CH2O- ? CH3CO2- + CH3CH2OH
4
Consider the following equilibrium:
CH3C CH + B ? CH3C
CH + B ? CH3C  C- + BH+
C- + BH+
Which of the following bases would favor products in this reaction?
A) OH-
B) CH3O-
C) NH3
D) NH2-
E) CH3CO2-
The weakest conjugate acid produces the strongest base (ammonia has a pKa of 36).
CH3C
 CH + B ? CH3C
CH + B ? CH3C  C- + BH+
C- + BH+Which of the following bases would favor products in this reaction?
A) OH-
B) CH3O-
C) NH3
D) NH2-
E) CH3CO2-
The weakest conjugate acid produces the strongest base (ammonia has a pKa of 36).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following is the strongest acid?
A) CH3CO2H
B) BrCH2CO2H
C) ClCH2CH2CO2H
D) ClCH2CH2CH2CO2H
E) CICH2CO2H
A) CH3CO2H
B) BrCH2CO2H
C) ClCH2CH2CO2H
D) ClCH2CH2CH2CO2H
E) CICH2CO2H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following is a factor in determining the acidity of a molecule?
A) electronegativity
B) size
C) hybridization
D) electron delocalization
E) all of the above
A) electronegativity
B) size
C) hybridization
D) electron delocalization
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
7
What form of glutamic acid (below) predominates in a solution of
PH = 7.2?
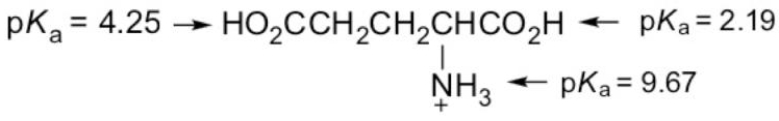
A)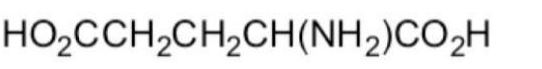
B)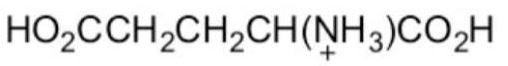
C)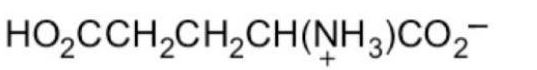
D)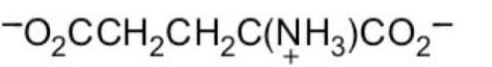
E)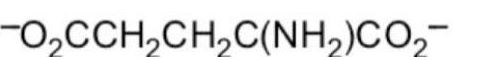
PH = 7.2?
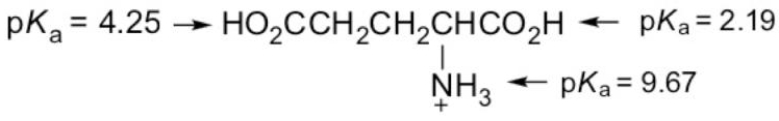
A)
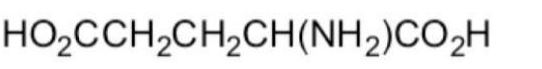
B)
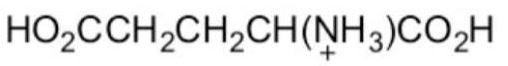
C)
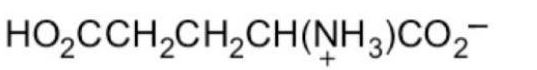
D)
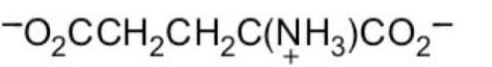
E)
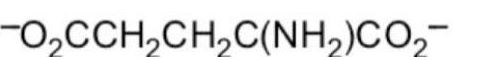

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following is not a Brønsted acid?
A) AlCl3
B) CH3OH
C) HNO3
D) BF3
E) A and D
A) AlCl3
B) CH3OH
C) HNO3
D) BF3
E) A and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following is a Lewis acid?
A) AlCl3
B) CH3OH
C) HNO3
D) BF3
E) all of the above
A) AlCl3
B) CH3OH
C) HNO3
D) BF3
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following is a Lewis base?
A) CH3NH3+
B) BF3
C) CH3O-
D) CH3OCH3
E) C and D
A) CH3NH3+
B) BF3
C) CH3O-
D) CH3OCH3
E) C and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 10 في هذه المجموعة.
فتح الحزمة
k this deck








