Deck 8: Delocalized Electrons Their Eff
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/11
العب
ملء الشاشة (f)
Deck 8: Delocalized Electrons Their Eff
1
Which of the following is not acceptable when drawing resonance structures?
A) Move electrons to sp or sp2 hybridized atoms.
B) Positive charges are moved toward electrons.
C) Move lone-pair electrons.
D) Never move atoms.
E) Move pi electrons.
A) Move electrons to sp or sp2 hybridized atoms.
B) Positive charges are moved toward electrons.
C) Move lone-pair electrons.
D) Never move atoms.
E) Move pi electrons.
Positive charges are moved toward electrons.
2
Which of the following is not an acceptable resonance structure of I?
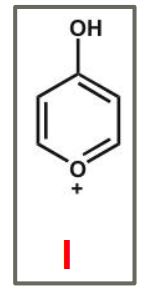
A)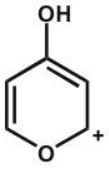
B)
C)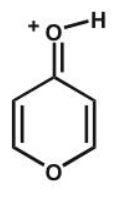
D)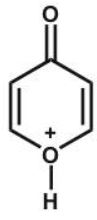
E)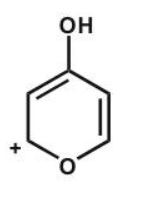

A)

B)

C)

D)

E)


3
Which structure makes the largest contribution to the resonance hybrid?
A)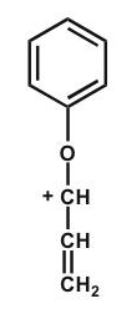
B)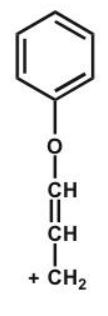
C)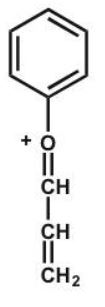
D)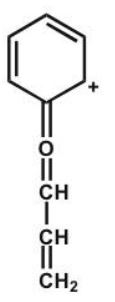
E)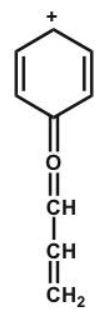
A)

B)

C)

D)

E)


4
Which of the following is the strongest acid?
A)
B)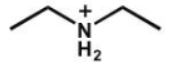
C)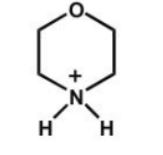
D)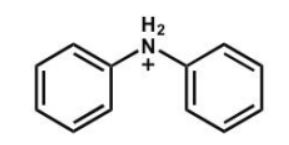
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following products is the result of either 1,2 or 1,4-addition of HBr and is most stable? 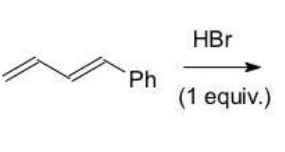
A)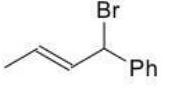
B)
C)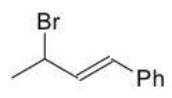
D)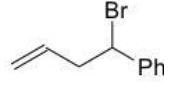
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which diene will react most readily with a dienophile?
A)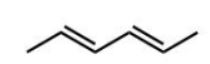
B)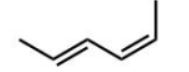
C)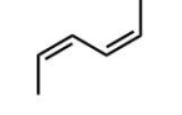
D)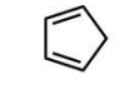
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which compound is the expected product of this reaction? 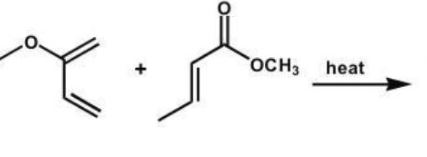
A)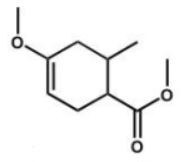
B)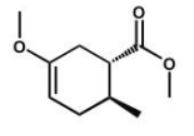
C)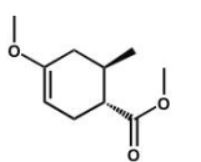
D)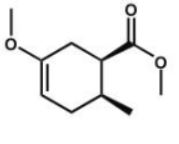
E)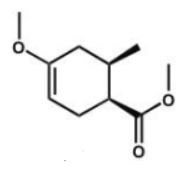

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following criteria must be met for a molecule to be aromatic?
A) The molecule must be cyclic.
B) The molecule must be planar.
C) Every atom must have a p orbital.
D) The molecule must contain an odd number of pairs of ? electrons.
E) All of the above.
A) The molecule must be cyclic.
B) The molecule must be planar.
C) Every atom must have a p orbital.
D) The molecule must contain an odd number of pairs of ? electrons.
E) All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which products are, respectively, the kinetic and thermodynamic product? 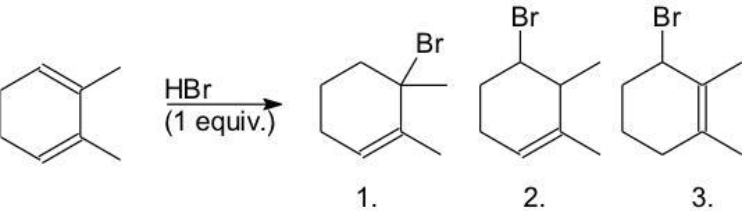
A) 1,2
B) 1,3
C) 2,1
D) 2,3
E) 3,1

A) 1,2
B) 1,3
C) 2,1
D) 2,3
E) 3,1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
10
Assuming planarity, which of the following molecules is aromatic?
A)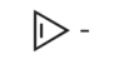
B)
C)
D)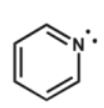
E)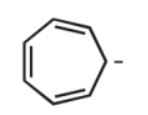
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following molecular orbital diagrams
best fits that of pyrrole?
pyrrole
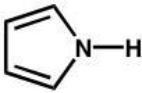
A)
B)
C)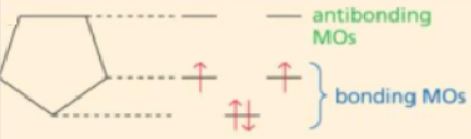
D)
best fits that of pyrrole?
pyrrole

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck








