Deck 6: Mechanisms of Enzymes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/88
العب
ملء الشاشة (f)
Deck 6: Mechanisms of Enzymes
1
Which mode(s)of catalysis is/are classified as chemical effects?
1. Transition state stabilization
2. Acid-base catalysis
3. Covalent catalysis
4. Proximity effect
A)3 only.
B)1,2 and 3.
C)2 and 3.
D)All of them.
1. Transition state stabilization
2. Acid-base catalysis
3. Covalent catalysis
4. Proximity effect
A)3 only.
B)1,2 and 3.
C)2 and 3.
D)All of them.
2 and 3.
2
The movement of ________ is key to understanding chemical and enzymatic reactions.
A)neutrons
B)electrons
C)nucleophiles
D)electrophiles
A)neutrons
B)electrons
C)nucleophiles
D)electrophiles
electrons
3
Site directed mutagenesis is used to study enzymes by
A)changing the location of the active site.
B)producing enzymes with different amino acid residues.
C)producing enzymes with modified R groups.
D)changing the pH of the environment.
A)changing the location of the active site.
B)producing enzymes with different amino acid residues.
C)producing enzymes with modified R groups.
D)changing the pH of the environment.
producing enzymes with different amino acid residues.
4
On the energy diagram below,which point represents an intermediate? 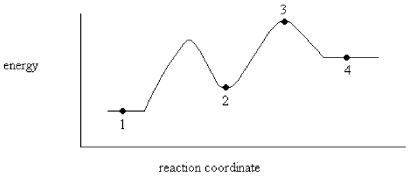
A)Point 1
B)Point 2
C)Point 3
D)Point 4

A)Point 1
B)Point 2
C)Point 3
D)Point 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
5
A chemical group that has a negative charge or an unshared electron pair may act as a(n)
A)transition state.
B)neutrophile.
C)electrophile.
D)nucleophile.
A)transition state.
B)neutrophile.
C)electrophile.
D)nucleophile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
6
Cleavage of a C-C bond produces a carbanion that ________ both electrons and a carbocation that ________ both electrons.
A)loses;loses
B)loses;keeps
C)keeps;loses
D)keeps;keeps
A)loses;loses
B)loses;keeps
C)keeps;loses
D)keeps;keeps

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
7
In the reaction below,Y- is ________. Y- + CH₂X → CH₂Y + X-
A)the leaving group
B)the attacking electrophile
C)the reaction intermediate
D)a nucleophile
A)the leaving group
B)the attacking electrophile
C)the reaction intermediate
D)a nucleophile

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
8
A detailed description of a chemical reaction in terms of the molecular,atomic or subatomic events is called the reaction ________.
A)mechanism
B)pathway
C)primary sequence
D)motif
A)mechanism
B)pathway
C)primary sequence
D)motif

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which represents a hydride ion?
A)H₂-
B)H-
C)H+
D)H₃O+
A)H₂-
B)H-
C)H+
D)H₃O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which will be different for a catalyzed reaction versus an uncatalyzed reaction?
A)Activation energy.
B)Ground state energy.
C)Free energy change.
D)All of the above.
A)Activation energy.
B)Ground state energy.
C)Free energy change.
D)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
11
How is the half-reaction Cu²+ + 2e- → Cu classified?
A)Free radical reaction.
B)Electrophilic substitution reaction.
C)Oxidation reaction.
D)Reduction reaction.
E)None of the above.
A)Free radical reaction.
B)Electrophilic substitution reaction.
C)Oxidation reaction.
D)Reduction reaction.
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many intermediates are indicated by the energy diagram below? 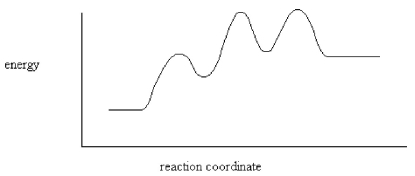
A)One
B)Two
C)Three
D)Four

A)One
B)Two
C)Three
D)Four

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
13
In this reaction,the carbon is ________. 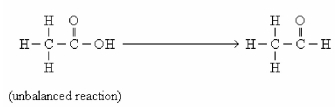
A)oxidized
B)reduced
C)neutralized
D)hydrolyzed

A)oxidized
B)reduced
C)neutralized
D)hydrolyzed

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which statement does not apply to transition states?
A)Many have been detected experimentally.
B)Chemical bonds are in the process of being formed and broken.
C)Have lifetimes on the order of 10-14 to 10-13 seconds.
D)Differ in energy from the ground state by the activation energy.
A)Many have been detected experimentally.
B)Chemical bonds are in the process of being formed and broken.
C)Have lifetimes on the order of 10-14 to 10-13 seconds.
D)Differ in energy from the ground state by the activation energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which amino acid is least likely to participate in acid-base catalysis?
A)Lysine.
B)Valine.
C)Aspartate.
D)Histidine.
A)Lysine.
B)Valine.
C)Aspartate.
D)Histidine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
16
In the following chemical reaction which species is the reducing agent?
CH₄ + 2 O2 → CO2 + 2 H₂O
A)CH₄
B)O2
C)CO2
D)H₂O
E)None of the above,this is not an oxidation-reduction reaction.
CH₄ + 2 O2 → CO2 + 2 H₂O
A)CH₄
B)O2
C)CO2
D)H₂O
E)None of the above,this is not an oxidation-reduction reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
17
On the energy diagram below,which point represents a transition state? 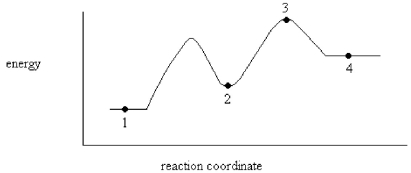
A)Point 1
B)Point 2
C)Point 3
D)Point 4

A)Point 1
B)Point 2
C)Point 3
D)Point 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
18
Replacement of the amino acid ________ at or near an active site of an enzyme is more likely to change enzyme activity than the replacement of ________ at or near the active site.
A)histidine;leucine
B)leucine;histidine
C)leucine;isoleucine
D)histidine;aspartate
A)histidine;leucine
B)leucine;histidine
C)leucine;isoleucine
D)histidine;aspartate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
19
On the energy diagram below,which arrow(s)represent the activation energy for the forward and reverse reactions? 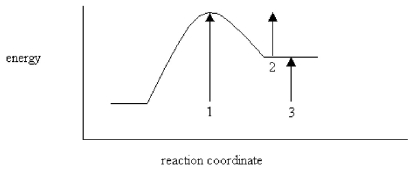
A)Arrow 1 is the activation energy for both the forward and reverse reactions.
B)Arrow 1 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.
C)Arrow 1 is the activation energy for the forward reaction and arrow 3 is the activation energy for the reverse reaction.
D)Arrow 3 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.

A)Arrow 1 is the activation energy for both the forward and reverse reactions.
B)Arrow 1 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.
C)Arrow 1 is the activation energy for the forward reaction and arrow 3 is the activation energy for the reverse reaction.
D)Arrow 3 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
20
An enzyme stabilizes the transition state that is bound in the active site.What effect will this have on the energy diagram below? The diagram shown below is for the uncatalyzed reaction. 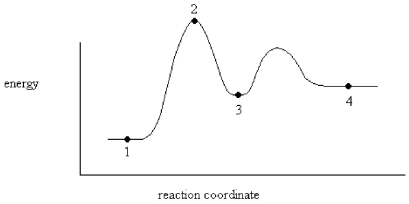
A)Energy of point 1 is raised.
B)Energy of point 2 is raised.
C)Energy of point 2 is lowered.
D)Energy of point 3 is lowered.
E)Energy of point 4 is raised.

A)Energy of point 1 is raised.
B)Energy of point 2 is raised.
C)Energy of point 2 is lowered.
D)Energy of point 3 is lowered.
E)Energy of point 4 is raised.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
21
The following pH dependence was found for the activity of a certain enzyme-catalyzed reaction. 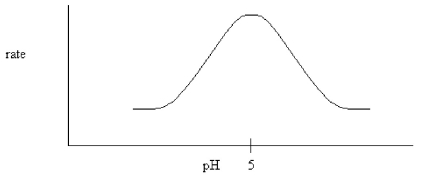
If it is known that the only two ionizable residues in the active site are both glutamates,which conclusion can be drawn?
A)The glutamates have different microenvironments which cause their pKa's to differ.
B)One of the glutamates must be amidated.
C)Both glutamates have a pKa equal to 5.0.
D)Both glutamates are deprotonated during the reaction.

If it is known that the only two ionizable residues in the active site are both glutamates,which conclusion can be drawn?
A)The glutamates have different microenvironments which cause their pKa's to differ.
B)One of the glutamates must be amidated.
C)Both glutamates have a pKa equal to 5.0.
D)Both glutamates are deprotonated during the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
22
When the concentration of a substrate inside a cell falls below the substrate concentration at half maximal velocity,________.
A)ES is formed more easily
B)ES dissociates to E + S
C)ES is closer to the ground state than to the transition state
D)E + S is in equilibrium with ES
E)the reaction will not proceed
A)ES is formed more easily
B)ES dissociates to E + S
C)ES is closer to the ground state than to the transition state
D)E + S is in equilibrium with ES
E)the reaction will not proceed

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
23
Superoxide dismutase enzyme catalysis is faster than the rate of diffusion because it
A)is an acid-base catalyst.
B)is a two-step reaction.
C)has an electric field around the active site.
D)occurs in very high quantities in cells.
A)is an acid-base catalyst.
B)is a two-step reaction.
C)has an electric field around the active site.
D)occurs in very high quantities in cells.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
24
A reaction that occurs with every collision between reactant molecules is called a(n)________.
A)saturation point
B)diffusion-controlled reaction
C)rate-determining step
D)first order reaction
A)saturation point
B)diffusion-controlled reaction
C)rate-determining step
D)first order reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
25
What shape would a graph of reaction velocity versus pH have for an enzyme that uses both a proton donor and a proton acceptor during catalysis (both acid and base catalysis)?
A)Sigmoidal.
B)Hyperbolic.
C)Exponential.
D)Bell-shaped.
E)Linear.
A)Sigmoidal.
B)Hyperbolic.
C)Exponential.
D)Bell-shaped.
E)Linear.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
26
The graph shows the rate of catalysis versus pH for an enzyme-catalyzed reaction.What conclusion can be made from the graph? 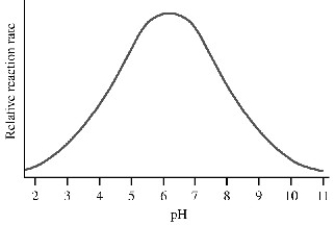
A)The enzyme is completely denatured by pHs greater than 8 or less than 5.
B)The substrate has four ionizable groups.
C)At pH 6.2 all ionizable groups in the active site are protonated.
D)The enzyme has an acidic and a basic amino acid in the active site.
E)The enzyme has only one protonatable residue in the active site.

A)The enzyme is completely denatured by pHs greater than 8 or less than 5.
B)The substrate has four ionizable groups.
C)At pH 6.2 all ionizable groups in the active site are protonated.
D)The enzyme has an acidic and a basic amino acid in the active site.
E)The enzyme has only one protonatable residue in the active site.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
27
In the nonpolar environment of most enzyme active sites,which statement applies to charge-charge interactions between the enzyme and the substrate?
A)They are rare due to the non-polar environment.
B)They are frequent,but not very strong in the nonpolar environment.
C)They are stronger in the nonpolar environment.
D)The polarity of the active site has no effect on the strength of the charge-charge interactions.
A)They are rare due to the non-polar environment.
B)They are frequent,but not very strong in the nonpolar environment.
C)They are stronger in the nonpolar environment.
D)The polarity of the active site has no effect on the strength of the charge-charge interactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
28
The reaction catalyzed by a certain phosphatase enzyme is found to follow ping-pong kinetics and involves the transfer of a phosphate group from substrate A to substrate B.Which mode of catalysis is likely for this reaction?
A)Sequential catalysis.
B)Acid-base catalysis.
C)Transfer catalysis.
D)Covalent catalysis.
A)Sequential catalysis.
B)Acid-base catalysis.
C)Transfer catalysis.
D)Covalent catalysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
29
Most Km values of enzymes for their substrates are on the order of ________ M.
A)10-2
B)10-3
C)10-4
D)10-5
E)10-6
A)10-2
B)10-3
C)10-4
D)10-5
E)10-6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
30
An enzyme's active site contains an arginine residue and a glutamate residue with pKa's of 2.9 and 9.1,respectively.Both residues are actively involved in the catalytic mechanism and they are the only two ionizable residues in the active site.What would you expect for the optimum pH of the enzyme?
A)4.0
B)6.0
C)8.0
D)No pH can be determined since the information is irrelevant to the optimum pH.
A)4.0
B)6.0
C)8.0
D)No pH can be determined since the information is irrelevant to the optimum pH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
31
The graphs below all represent the same chemical reaction,but each employing a different catalyst.Which enzyme uses the most efficient mechanism of catalysis? 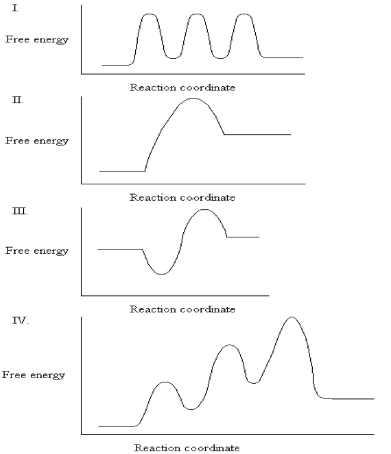
A)See Graph I.
B)See Graph II.
C)See Graph III.
D)See Graph IV.

A)See Graph I.
B)See Graph II.
C)See Graph III.
D)See Graph IV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
32
Histidine is an ideal amino acid at neutral pH values at the active site of many enzymes because
A)it is hydrophobic.
B)it engages in electron transfer.
C)it is not ionizable.
D)its R group has a pKa of about 6 to 7 in most proteins.
A)it is hydrophobic.
B)it engages in electron transfer.
C)it is not ionizable.
D)its R group has a pKa of about 6 to 7 in most proteins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which graph might you expect for the pH profile of an enzyme's activity if the only ionizable residue in the active site is aspartate? 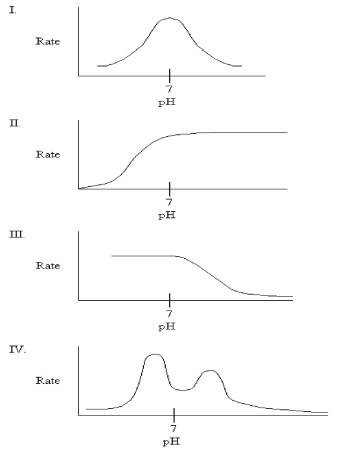
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
34
SOD (superoxide dismutase)is an enzyme that reacts faster than the rate of diffusion of the substrate to the active site due to
A)its negative charge.
B)electrostatic effects.
C)the deep channel in the enzyme protein.
D)the copper atom at the active site.
E)All of the above.
A)its negative charge.
B)electrostatic effects.
C)the deep channel in the enzyme protein.
D)the copper atom at the active site.
E)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
35
A histidine was determined to be the critical residue involved in an enzyme-catalyzed reaction.If the pKa of the histidine is known to be 6.5 in the active site and the pH of maximum catalytic activity is 7.2,what is likely the primary role of histidine in the catalytic reaction?
A)Acts as a proton donor or acceptor.
B)Forms a covalent bond with the substrate.
C)Stabilizes a charged intermediate.
D)Reduces the entropy of the substrate.
A)Acts as a proton donor or acceptor.
B)Forms a covalent bond with the substrate.
C)Stabilizes a charged intermediate.
D)Reduces the entropy of the substrate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
36
A thermodynamic pit occurs when
A)ES is not very stable.
B)ES forms faster than it dissociates.
C)ES is highly stable.
D)S is not bound tightly to an enzyme.
E)S is positioned incorrectly to the enzyme.
A)ES is not very stable.
B)ES forms faster than it dissociates.
C)ES is highly stable.
D)S is not bound tightly to an enzyme.
E)S is positioned incorrectly to the enzyme.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
37
An update of Fischer's lock-and-key theory of enzyme specificity views the ________ as the lock and ________ as the key.
A)enzyme;substrate
B)substrate;enzyme
C)enzyme;transition state
D)transition state;enzyme
E)substrate;transition state
A)enzyme;substrate
B)substrate;enzyme
C)enzyme;transition state
D)transition state;enzyme
E)substrate;transition state

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
38
Aspartate and lysine are in the active site of an enzyme.They are both known to participate directly in catalysis.The pKa's of the residues are found to be 3.2 and 9.6,respectively for aspartate and lysine.The optimum pH for the enzyme is 6.4.Which forms of these two residues will predominate when the enzyme is most active?
A)Aspartate is protonated;lysine is deprotonated.
B)Both residues are protonated.
C)Aspartate is deprotonated;lysine is protonated.
D)Both residues are deprotonated.
A)Aspartate is protonated;lysine is deprotonated.
B)Both residues are protonated.
C)Aspartate is deprotonated;lysine is protonated.
D)Both residues are deprotonated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
39
The active site of a certain enzyme contains a serine residue.When the enzyme is incubated for a short time with its substrate,a form of the enzyme in which the active site serine is acetylated can be isolated and purified.In the native protein the serine is never found to be acetylated.This information supports ________.
A)a covalent catalysis mode
B)intermediate state stabilization effects
C)the acid-base catalysis mode
D)a polar group catalytic mechanism
A)a covalent catalysis mode
B)intermediate state stabilization effects
C)the acid-base catalysis mode
D)a polar group catalytic mechanism

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
40
Acid-base catalysis is estimated to accelerate a typical enzymatic reaction by what factor?
A)1 to 2 fold increase.
B)10 to 100 fold increase.
C)106 fold increase.
D)1023 fold increase.
A)1 to 2 fold increase.
B)10 to 100 fold increase.
C)106 fold increase.
D)1023 fold increase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
41
One reason the proximity effect enhances catalysis is because
A)the effective molarity of reactive substrate groups increases.
B)the enzyme changes conformation to more readily accept the substrate as it approaches the active site.
C)the active site becomes smaller.
D)the catalytic triad in the active site becomes more flexible.
A)the effective molarity of reactive substrate groups increases.
B)the enzyme changes conformation to more readily accept the substrate as it approaches the active site.
C)the active site becomes smaller.
D)the catalytic triad in the active site becomes more flexible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
42
A key role of the hydroxyl group at position 6 in the purine ring in the formation of a transition state by the enzyme adenosine deaminase is obtained by comparing a ________ and a ________.
A)competitive inhibitor;noncompetitive inhibitor
B)transition state analog;normal substrate
C)noncompetitive inhibitor;transition state analog
D)competitive inhibitor;transition state analog
E)All of the above.
A)competitive inhibitor;noncompetitive inhibitor
B)transition state analog;normal substrate
C)noncompetitive inhibitor;transition state analog
D)competitive inhibitor;transition state analog
E)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which bond in the polysaccharide shown is cleaved by lysozyme? 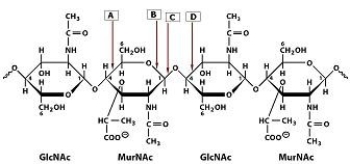
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
44
The role of serine at the active site of serine proteases is to act as a(n)________ catalyst,while the histidine residue serves as a(n)________ catalyst.
A)strong;weak
B)weak;strong
C)acid-base;covalent
D)covalent;acid-base
E)anionic;ionic
A)strong;weak
B)weak;strong
C)acid-base;covalent
D)covalent;acid-base
E)anionic;ionic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
45
Transition state analogs should
A)stabilize transition states.
B)have a dissociation constant of 10-¹³ M or less.
C)bind very tightly to the enzyme.
D)A and C.
E)B and C.
A)stabilize transition states.
B)have a dissociation constant of 10-¹³ M or less.
C)bind very tightly to the enzyme.
D)A and C.
E)B and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
46
Induced fit studies by Koshland using the enzyme hexokinase showed that there are enzyme forms
A)which react with the hydroxyl group of water.
B)which react only with ATP and glucose present together.
C)which are hydrophobic,excluding the competing hydroxyl group from water.
D)with and without glucose bound to each.
E)that hydrolyze ATP.
A)which react with the hydroxyl group of water.
B)which react only with ATP and glucose present together.
C)which are hydrophobic,excluding the competing hydroxyl group from water.
D)with and without glucose bound to each.
E)that hydrolyze ATP.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
47
Experiments on the bacterial serine protease subtilisin show that even when all three residues of the catalytic triad are mutated,the catalytic rate of the enzyme is still 3000 times the uncatalyzed reaction rate.Which mode of catalysis is likely responsible for this remaining catalytic activity?
A)Acid-base catalysis.
B)Covalent catalysis.
C)Transition-state stabilization.
D)Hydrophobic effects.
A)Acid-base catalysis.
B)Covalent catalysis.
C)Transition-state stabilization.
D)Hydrophobic effects.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
48
The mechanism of action of lysozyme includes
A)distortion of the substrate.
B)acid catalysis.
C)proximity effects.
D)formation of a half-chair sugar form.
E)All of the above.
A)distortion of the substrate.
B)acid catalysis.
C)proximity effects.
D)formation of a half-chair sugar form.
E)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
49
Glycoside hydrolases such as bacterial cellulase have been shown to differ in mechanisms from that of lysozyme.They
A)are alkaline catalysts.
B)form a boat form of the sugar.
C)form a covalent sugar-enzyme intermediate.
D)do not distort the substrate.
E)have no active site side chain interactions.
A)are alkaline catalysts.
B)form a boat form of the sugar.
C)form a covalent sugar-enzyme intermediate.
D)do not distort the substrate.
E)have no active site side chain interactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
50
The enzyme has an active site which
A)fits the substrate exactly.
B)fits the transition state.
C)may contain hydrogen bonds which are covalent-like.
D)A and C.
E)B and C.
A)fits the substrate exactly.
B)fits the transition state.
C)may contain hydrogen bonds which are covalent-like.
D)A and C.
E)B and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
51
The substrate specificity of serine proteases is primarily due to
A)a specificity pocket in the protein.
B)the positions of specific side chains of serine,histidine,and aspartate.
C)distinct backbone conformations of the individual proteins.
D)A and B.
E)A,B and C.
A)a specificity pocket in the protein.
B)the positions of specific side chains of serine,histidine,and aspartate.
C)distinct backbone conformations of the individual proteins.
D)A and B.
E)A,B and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
52
Zymogens are inactive enzyme precursors which are made active by
A)a change in folding only.
B)selective proteolysis.
C)methylation of particular residues.
D)a change in pH.
A)a change in folding only.
B)selective proteolysis.
C)methylation of particular residues.
D)a change in pH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the biological function of lysozyme?
A)Converts trypsinogen to trypsin.
B)Is a ligase that accelerates the polymerization of glycogen.
C)It regulates vascular constriction in chickens.
D)Hydrolyzes polysaccharides of bacterial cell walls.
A)Converts trypsinogen to trypsin.
B)Is a ligase that accelerates the polymerization of glycogen.
C)It regulates vascular constriction in chickens.
D)Hydrolyzes polysaccharides of bacterial cell walls.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
54
The role of ser-195 in chymotrypsin cleavage of a peptide bond is that of a(n)
A)acid catalyst.
B)proximity effector.
C)strong nucleophile.
D)weak nucleophile.
A)acid catalyst.
B)proximity effector.
C)strong nucleophile.
D)weak nucleophile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
55
The proximity effect of speeding an enzyme-catalyzed reaction is explained by ________.
A)a large loss of entropy when reactive groups are brought close to each other
B)a large gain in entropy due to binding of the substrate
C)a conversion of a reaction from endergonic to exergonic
D)increasing the flexibility of the substrate by increasing its degrees of freedom of rotation
A)a large loss of entropy when reactive groups are brought close to each other
B)a large gain in entropy due to binding of the substrate
C)a conversion of a reaction from endergonic to exergonic
D)increasing the flexibility of the substrate by increasing its degrees of freedom of rotation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
56
The roles of amino acid residues at the active site of enzymes can be determined by removing certain residues using the technique of
A)specific hydrolysis.
B)covalent binding.
C)acylation of specific residues.
D)site-directed mutagenesis.
E)All of the above.
A)specific hydrolysis.
B)covalent binding.
C)acylation of specific residues.
D)site-directed mutagenesis.
E)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
57
Pepsin and trypsin are produced in the pancreas as zymogens.What might result if these two enzymes were not produced in the pancreas as zymogens,but as active pepsin and trypsin?
A)They would not be transported to the intestine because of their native charge.
B)They would not be folded properly by their chaperone molecules.
C)They would associate into inactive oligomeric forms.
D)They would begin to degrade and damage pancreatic proteins.
A)They would not be transported to the intestine because of their native charge.
B)They would not be folded properly by their chaperone molecules.
C)They would associate into inactive oligomeric forms.
D)They would begin to degrade and damage pancreatic proteins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
58
Active trypsin formation by the action of enteropeptidase can be viewed as the master activation step because
A)enteropeptidase can activate its own zymogen.
B)it is allosterically controlled.
C)trypsin activates other pancreatic zymogens.
D)All of the above.
A)enteropeptidase can activate its own zymogen.
B)it is allosterically controlled.
C)trypsin activates other pancreatic zymogens.
D)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
59
The catalytic triad of chymotrypsin and other serine proteases consists of
A)three subunits of the enzyme.
B)three amino acid residues adjacent in the primary structure which act to make serine a strong nucleophile.
C)three amino acid residues close enough in space to make serine a strong nucleophile.
D)three enzymes with very similar structural features.
E)None of the above.
A)three subunits of the enzyme.
B)three amino acid residues adjacent in the primary structure which act to make serine a strong nucleophile.
C)three amino acid residues close enough in space to make serine a strong nucleophile.
D)three enzymes with very similar structural features.
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
60
The induced fit model of enzyme activation includes
A)contact (binding)of substrate.
B)constant small and rapid motions of protein atoms.
C)conversion of the enzyme to an active form.
D)A and B.
E)A,B,and C.
A)contact (binding)of substrate.
B)constant small and rapid motions of protein atoms.
C)conversion of the enzyme to an active form.
D)A and B.
E)A,B,and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
61
Superoxide dismutase and triosephosphate isomerase are two enzymes that catalyze diffusion-controlled reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
62
Radicals are stable,unreactive species that have an unpaired electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
63
Reaction intermediates are impossible to isolate experimentally.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
64
A nucleophile is an electron poor species.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
65
Chemical modes of catalysis are more important than binding modes of catalysis in accounting for the accelerated rates of enzymatic reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
66
The relative orientation of colliding molecules only affects reactions rates when the energy during impact is low.If the energy of impact is high enough,orientation is unimportant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
67
X-ray crystallographic examination of the active site of arginine kinase was possible because
A)it could be crystallized in the different structural forms.
B)nitrate could be substituted for phosphate in the reaction.
C)it was readily purified (unlike most other enzymes).
D)MgADP was involved in the reaction.
E)All of the above.
A)it could be crystallized in the different structural forms.
B)nitrate could be substituted for phosphate in the reaction.
C)it was readily purified (unlike most other enzymes).
D)MgADP was involved in the reaction.
E)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
68
Transition state analogs are usually more potent inhibitors of enzyme activity than substrate analogs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which statement describes the mechanism of lysozyme's catalyzed reaction?
A)It includes substrate distortion and stabilization of an unstable oxocarbocation intermediate.
B)It requires lysozyme to be phosphorylated before catalysis can occur.
C)The catalyzed reaction produces three important isolatable intermediates by converting the reaction into a four-step mechanism.
D)All of the above.
A)It includes substrate distortion and stabilization of an unstable oxocarbocation intermediate.
B)It requires lysozyme to be phosphorylated before catalysis can occur.
C)The catalyzed reaction produces three important isolatable intermediates by converting the reaction into a four-step mechanism.
D)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
70
Unlike lysozyme,other glycoside hydrolases such as bacterial cellulase,form covalent glycosyl-enzyme intermediates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
71
Transitions states bind to their enzymes more tightly than their substrates do.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
72
If a plot of enzyme activity versus pH is sigmoidal then acid-base catalysis can be ruled out as a mode of catalysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
73
Zymogens are often active only in the environment in which they are functional.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
74
Enzyme-catalyzed reactions that are diffusion-controlled reactions can never proceed any faster than the rate determined by random collision rates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
75
Weak substrate binding is an important feature to help describe enzyme catalysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
76
In the modified "lock-and-key" theory of enzyme specificity,the key is still the enzyme while the lock is still the substrate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
77
Enzymes which have induced fit to the substrate are generally more effective than those in an active form initially.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
78
Intermediates are more stable and have longer lifetimes than transition states.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
79
Lysozyme is classified as a
A)Phosphorylase.
B)Oxidoreductase.
C)Isomerase.
D)Transferase.
E)Hydrolase.
A)Phosphorylase.
B)Oxidoreductase.
C)Isomerase.
D)Transferase.
E)Hydrolase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
80
Nucleophiles are often anions or have unshared electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck








