Deck 9: Covalent Bonding and Molecules
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/30
العب
ملء الشاشة (f)
Deck 9: Covalent Bonding and Molecules
1
A ClO2- ion has _____ valence electrons.
A) 16
B) 18
C) 20
D) 22
A) 16
B) 18
C) 20
D) 22
20
2
Which Lewis structure,although acceptable,LEAST describes the carbonate ion? 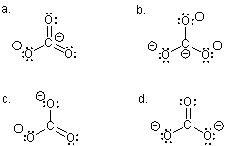
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d
b
3
Which compound has an expanded octet around the central atom?
A) NH3
B) PCl3
C) SF6
D) CF4
A) NH3
B) PCl3
C) SF6
D) CF4
SF6
4
What is the formal charge on the nitrogen atom in this structure? 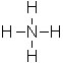
A) +1
B) +2
C) -1
D) -3

A) +1
B) +2
C) -1
D) -3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
5
Ozone has the formula O3.Which statement is CORRECT concerning ozone?
A) One oxygen atom has a +1 formal charge while another has a -1 formal charge.The third one is neutral.
B) All three atoms are neutral.
C) Two atoms have a -1 formal charge,and the third oxygen atom has a +1 formal charge.
D) Two atoms have a +1 formal charge,and the third oxygen atom has a -1 formal charge.
A) One oxygen atom has a +1 formal charge while another has a -1 formal charge.The third one is neutral.
B) All three atoms are neutral.
C) Two atoms have a -1 formal charge,and the third oxygen atom has a +1 formal charge.
D) Two atoms have a +1 formal charge,and the third oxygen atom has a -1 formal charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
6
How many atoms in this molecule have a tetrahedral geometry? 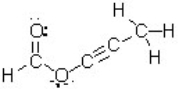
A) one
B) two
C) three
D) four

A) one
B) two
C) three
D) four

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
7
How many atoms in this molecule have a trigonal planar geometry? 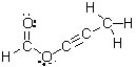
A) one
B) two
C) three
D) four

A) one
B) two
C) three
D) four

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the electronic geometry around the central atom in CO2? 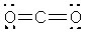
A) trigonal planar
B) linear
C) tetrahedral
D) bent
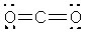
A) trigonal planar
B) linear
C) tetrahedral
D) bent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
9
The Lewis structure of the cyanide ion is shown here.How many valence electrons are in this ion? 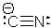
A) 11
B) 10
C) 8
D) 7
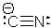
A) 11
B) 10
C) 8
D) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which molecule does NOT have a lone pair on the central atom?
A) NH3
B) SF2
C) PCl3
D) CO2
A) NH3
B) SF2
C) PCl3
D) CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which statement is CORRECT concerning the cyanide ion? 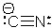
A) The carbon atom has a tetrahedral geometry,and the nitrogen atom has a liner geometry.
B) The carbon atom has a trigonal planar geometry,and the nitrogen atom has a liner geometry.
C) Both atoms have a linear geometry.
D) Both atoms have a trigonal planar geometry.
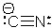
A) The carbon atom has a tetrahedral geometry,and the nitrogen atom has a liner geometry.
B) The carbon atom has a trigonal planar geometry,and the nitrogen atom has a liner geometry.
C) Both atoms have a linear geometry.
D) Both atoms have a trigonal planar geometry.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
12
A phosphorus atom with four bonds has a charge of:
A) +1.
B) +2.
C) -1.
D) -2.
A) +1.
B) +2.
C) -1.
D) -2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the formal charge on the oxygen atom in this structure? 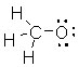
A) +1
B) +2
C) -1
D) -2

A) +1
B) +2
C) -1
D) -2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
14
In this Lewis structure,which atom does NOT have a complete valence? 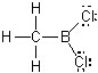
A) H
B) B
C) C
D) Cl

A) H
B) B
C) C
D) Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which Lewis structure BEST describes the carbonate ion? 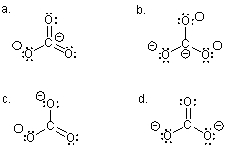
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which compound has 24 valence electrons?
A) C3H6O
B) C2H6O
C) CO2
D) HCN
A) C3H6O
B) C2H6O
C) CO2
D) HCN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
17
A neutral carbon atom has _____ valence electrons.
A) two
B) four
C) five
D) eight
A) two
B) four
C) five
D) eight

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
18
A oxygen atom with one bond and three lone pairs has a charge of:
A) +1.
B) +2.
C) -1.
D) -2.
A) +1.
B) +2.
C) -1.
D) -2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
19
A neutral sulfur atom has _____ valence electrons.
A) two
B) four
C) six
D) eight
A) two
B) four
C) six
D) eight

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the electronic geometry around the oxygen atoms in CO2? 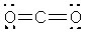
A) trigonal planar
B) linear
C) tetrahedral
D) bent
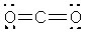
A) trigonal planar
B) linear
C) tetrahedral
D) bent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which atom is the MOST electronegative?
A) Ca
B) Sr
C) Ba
D) Be
A) Ca
B) Sr
C) Ba
D) Be

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which structure BEST describes the polarity of an H-O bond? 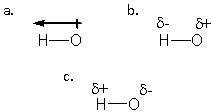
A) a
B) b
C) c

A) a
B) b
C) c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which molecule(s)is/are likely to be a gas at room temperature due to a lack of attraction to another molecule of its own kind? 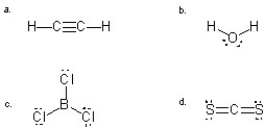
A) a,b,and c
B) a,c,and d
C) b
D) c and d
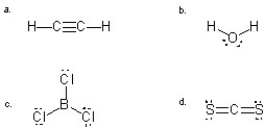
A) a,b,and c
B) a,c,and d
C) b
D) c and d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the correct ELECTRONIC GEOMETRY and MOLECULAR GEOMETRY around the central nitrogen in NO2-? (Hint: You may need to draw the molecule.)
A) tetrahedral,trigonal pyramidal
B) tetrahedral,tetrahedral
C) linear,linear
D) trigonal planar,bent
A) tetrahedral,trigonal pyramidal
B) tetrahedral,tetrahedral
C) linear,linear
D) trigonal planar,bent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which molecule has a NET DIPOLE? 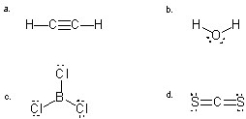
A) a
B) b
C) c
D) d
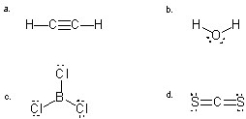
A) a
B) b
C) c
D) d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
26
Based on the Pauling electronegativities,an Fr-F bond is:
A) ionic.
B) covalent.
C) polar ionic.
D) polar covalent.
A) ionic.
B) covalent.
C) polar ionic.
D) polar covalent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
27
Based on the Pauling electronegativities,a C-H bond is:
A) ionic.
B) covalent.
C) polar ionic.
D) polar covalent.
A) ionic.
B) covalent.
C) polar ionic.
D) polar covalent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which molecule will have the STRONGEST attraction to another molecule of its own kind? 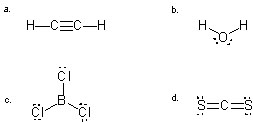
A) a
B) b
C) c
D) d
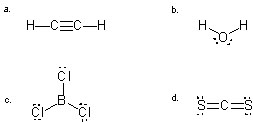
A) a
B) b
C) c
D) d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
29
Based on the Pauling electronegativities,a P-Cl bond is:
A) ionic.
B) covalent.
C) polar ionic.
D) polar covalent.
A) ionic.
B) covalent.
C) polar ionic.
D) polar covalent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which atom is the MOST electronegative?
A) Li
B) C
C) P
D) O
A) Li
B) C
C) P
D) O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 30 في هذه المجموعة.
فتح الحزمة
k this deck








