Deck 4: Chemical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/170
العب
ملء الشاشة (f)
Deck 4: Chemical Reactions
1
In a balanced equation,the number of atoms of each element in the products must equal to the number of atoms of each element in the reactants.
True
2
When the equation CS2 + Cl2 → CCl4 + S2Cl2 is balanced with the smallest integer coefficients,the sum of the coefficients is:
A)5
B)6
C)4
D)3
E)7
A)5
B)6
C)4
D)3
E)7
6
3
How many moles of H3PO4 are produced when 20.0 g of HCl are produced by the reaction 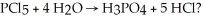
A)(20.0/36.5)g
B)(20.0/35.5)/5 g
C)(20.0/36.5)/5 g
D)(20.0/98.0)g
E)(20.0/98.0)/5 g
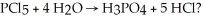
A)(20.0/36.5)g
B)(20.0/35.5)/5 g
C)(20.0/36.5)/5 g
D)(20.0/98.0)g
E)(20.0/98.0)/5 g
(20.0/36.5)/5 g
4
How many grams of N2 are required to react with 2.30 moles of Mg in the following process?
3 Mg + N2 → Mg3N2? (Mg = 24.3 g/mol,N = 14.0 g/mol)
A)21.5 g
B)0.767 g
C)64.4 g
D)0.027 g
E)193.2 g
3 Mg + N2 → Mg3N2? (Mg = 24.3 g/mol,N = 14.0 g/mol)
A)21.5 g
B)0.767 g
C)64.4 g
D)0.027 g
E)193.2 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
5
In a solution of alcohol and water that is 70% water,alcohol is the solvent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
6
Molarity is defined as moles of solute per kg of solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
7
A formula is a shorthand way of representing a chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
Na + HCl → NaCl + H2
A)7
B)4
C)2
D)10
E)9
Na + HCl → NaCl + H2
A)7
B)4
C)2
D)10
E)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
9
When the equation Fe2(C2O4)3 → FeC2O4 + CO2 is balanced with the smallest integer coefficients,the coefficient of CO2 is:
A)1
B)2
C)4
D)3
E)5
A)1
B)2
C)4
D)3
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
10
In a chemical equation,Δ above the yield sign means the reaction will produce heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
11
(aq)indicates that the compound is dissolved in alcohol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
12
A chemical equation is a shorthand way of representing a chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the sum of the coefficients in the balanced equation that represents the complete combustion of the relatively new gasoline additive "MTBE," for which the molecular formula is C5H12O?
A)39
B)37
C)29
D)24
E)20
A)39
B)37
C)29
D)24
E)20

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
14
The reactant that is in excess determines the amount of products formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
15
The numbers in front of formulas in balanced equations are called stoichiometric coefficients.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
16
Stoichiometric factor relates the amounts,in moles,of any two substances involved in chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which metal will produce the most hydrogen per gram of metal?
A)2 Li + 2 HCl → 2 LiCl + H2
B)Sn + 4 HCl → SnCl4 + 2 H2
C)2 Fe + 6 HCl →2 FeCl3 + 3 H2
D)Mg + 2 HCl → MgCl2 + H2
E)2 Cr + 6 HCl → 2 CrCl3 + 3H2
A)2 Li + 2 HCl → 2 LiCl + H2
B)Sn + 4 HCl → SnCl4 + 2 H2
C)2 Fe + 6 HCl →2 FeCl3 + 3 H2
D)Mg + 2 HCl → MgCl2 + H2
E)2 Cr + 6 HCl → 2 CrCl3 + 3H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
18
For the reaction 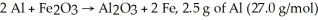
And 7.2 g of Fe2O3 (159.8 g/mol)produce how many g of Fe (55.9 g/mol)?
A)2.5 (55.9/27.0)g
B)2.5 (55.9)(2)/(27.0)(2)g
C)7.2 (55.9)(2)/159.8 g
D)7.2 (55.9/159.8)g
E)2.5 (55.9/159.8)g
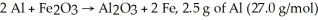
And 7.2 g of Fe2O3 (159.8 g/mol)produce how many g of Fe (55.9 g/mol)?
A)2.5 (55.9/27.0)g
B)2.5 (55.9)(2)/(27.0)(2)g
C)7.2 (55.9)(2)/159.8 g
D)7.2 (55.9/159.8)g
E)2.5 (55.9/159.8)g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
19
When the equation K2S2O3 + I2 → K2S4O6 + KI is balanced with the smallest integer coefficients,the coefficient of KI is:
A)2
B)1
C)3
D)4
E)5
A)2
B)1
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
K2O + H2O → KOH
A)2
B)6
C)3
D)8
E)4
K2O + H2O → KOH
A)2
B)6
C)3
D)8
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
21
What volume of 2.0 M HCl,in mL,is required to dissolve a 10.0 g piece of Zn?
Zn(s)+ 2 HCl → ZnCl2 + H2(g)
A)76 mL
B)330 mL
C)170 mL
D)310 mL
E)150 mL
Zn(s)+ 2 HCl → ZnCl2 + H2(g)
A)76 mL
B)330 mL
C)170 mL
D)310 mL
E)150 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
22
Potassium superoxide (KO2)can simulate a plant-type action by consuming carbon dioxide (CO2)gas and releasing oxygen (O2)gas.The other product is potassium carbonate (K2CO3).When the equation for this process is balanced,it shows that:
A)3 mol oxygen is produced per mol KO2 consumed
B)2 mol KO2 is consumed per mol carbon dioxide
C)moles of reactants equals moles of product
D)3 g of oxygen is produced per 2 g CO2 consumed
E)moles of products exceed moles of reactants
A)3 mol oxygen is produced per mol KO2 consumed
B)2 mol KO2 is consumed per mol carbon dioxide
C)moles of reactants equals moles of product
D)3 g of oxygen is produced per 2 g CO2 consumed
E)moles of products exceed moles of reactants

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
23
If 5.97 mL of a solution of NaCl contains 2.54 mg of sodium ion,what is the molarity of the sodium chloride solution?
A)0.425 M
B)1.85 × 10-2 M
C)1.85 × 10-5 M
D)7.28 × 10-3 M
E)0.102 M
A)0.425 M
B)1.85 × 10-2 M
C)1.85 × 10-5 M
D)7.28 × 10-3 M
E)0.102 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following represents a 1.00 M aqueous solution of glucose (C6H12O6)?
A)90.0 g glucose per 500 mL water
B)10.0 g glucose per 10.0 mL water
C)0.180 g glucose per mL solution
D)0.100 g glucose per mL solution
E)4.5 g glucose per 4.5 g water
A)90.0 g glucose per 500 mL water
B)10.0 g glucose per 10.0 mL water
C)0.180 g glucose per mL solution
D)0.100 g glucose per mL solution
E)4.5 g glucose per 4.5 g water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
25
Given the following reaction:
Na2SO4(s)+ 2 C(s)→ Na2S(s)+ 2 CO2(g)
How many grams of carbon are required to produce 18.4 g Na2S(s)?
A)11.3 g
B)5.66 g
C)2.83 g
D)239 g
E)142 g
Na2SO4(s)+ 2 C(s)→ Na2S(s)+ 2 CO2(g)
How many grams of carbon are required to produce 18.4 g Na2S(s)?
A)11.3 g
B)5.66 g
C)2.83 g
D)239 g
E)142 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
26
If 85.6 mL of a 6.75 M solution are diluted to 6.20 L with water,what is the concentration of the final solution?
A)6.75 (6.20/85.6)M
B)6.75 (8.56/6.20)M
C)6.75 (6200/85.6)M
D)6.75 (85.6/6200)M
E)8.56 (6.20/6.75)M
A)6.75 (6.20/85.6)M
B)6.75 (8.56/6.20)M
C)6.75 (6200/85.6)M
D)6.75 (85.6/6200)M
E)8.56 (6.20/6.75)M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
27
Given the reaction:
2KMnO4 + 10 KI + 8 H2SO4 → 6 K2SO4 + 2 MnSO4 + 5 I2 + 8 H2O
How many moles of H2SO4 are required to produce 2.0 moles of I2,given the other reactants are in excess?
A)0.80 mol
B)1.3 mol
C)3.2 mol
D)4.0 mol
E)1.6 mol
2KMnO4 + 10 KI + 8 H2SO4 → 6 K2SO4 + 2 MnSO4 + 5 I2 + 8 H2O
How many moles of H2SO4 are required to produce 2.0 moles of I2,given the other reactants are in excess?
A)0.80 mol
B)1.3 mol
C)3.2 mol
D)4.0 mol
E)1.6 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
28
45.8 mL of a 3.14 M sodium chloride solution were used to react completely with 50.0 mL of an aqueous silver nitrate solution.What is the molarity of the silver nitrate solution?
A)2.88 M
B)1.50 M
C)3.14 M
D)3.42 M
E)1.71 M
A)2.88 M
B)1.50 M
C)3.14 M
D)3.42 M
E)1.71 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
29
52.5 mL of a solution were diluted to a volume of 6.25 L and then had a concentration of 3.16 M.What was the molarity of the initial solution?
A)3.16(52.5/6.25)M
B)3.16(6.25/52.5)M
C)(3.16)(52.5)(6.25)M
D)3.16(52.5/6250)M
E)(6.25)(3.16)/0.0525 M
A)3.16(52.5/6.25)M
B)3.16(6.25/52.5)M
C)(3.16)(52.5)(6.25)M
D)3.16(52.5/6250)M
E)(6.25)(3.16)/0.0525 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
30
What volume of 6.0 M sulfuric acid is required for the preparation of 500.0 mL of 0.30 M solution?
A)100 mL
B)50 mL
C)40 mL
D)30 mL
E)25 mL
A)100 mL
B)50 mL
C)40 mL
D)30 mL
E)25 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following processes could theoretically produce the maximum mass of oxygen per gram of metal salt reactant?
A)2 KClO3 → 2 KCl + 3 O2
B)4 KO2 + 2 CO2 → 2 K2CO3 + 3 O2
C)2 Na2O2 + 2 H2SO4 → 2 H2O + O2
D)2 HgO → 2 Hg + O2
E)NaBrO2 → NaBr + O2
A)2 KClO3 → 2 KCl + 3 O2
B)4 KO2 + 2 CO2 → 2 K2CO3 + 3 O2
C)2 Na2O2 + 2 H2SO4 → 2 H2O + O2
D)2 HgO → 2 Hg + O2
E)NaBrO2 → NaBr + O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
32
A 1.900 g sample of C6H12 is burned in an excess of oxygen.What mass of CO2 and H2O should be obtained?
A)0.994 g CO2,0.407 g H2O
B)2.98 g CO2,1.22 g H2O
C)5.96 g CO2,2.44 g H2O
D)10.45 g CO2,4.27 g G H2O
E)5.23 g CO2,2.38 g H2O
A)0.994 g CO2,0.407 g H2O
B)2.98 g CO2,1.22 g H2O
C)5.96 g CO2,2.44 g H2O
D)10.45 g CO2,4.27 g G H2O
E)5.23 g CO2,2.38 g H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
33
24.0 g of ethane (C2H6)are burned to form CO2 and H2O.How many grams of CO2 are produced?
A)32.8 g
B)14.4 g
C)43.2 g
D)35.1 g
E)70.3 g
A)32.8 g
B)14.4 g
C)43.2 g
D)35.1 g
E)70.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
34
Iron metal reacts with chlorine gas as follows:
2 Fe(s)+ 3 Cl2(g)→ 2 FeCl3(s)
How many moles of FeCl3 are obtained when 4.6 mol of Cl2 reacts with excess Fe?
A)3.1 mol
B)4.6 mol
C)1.5 mol
D)2.3 mol
E)6.9 mol
2 Fe(s)+ 3 Cl2(g)→ 2 FeCl3(s)
How many moles of FeCl3 are obtained when 4.6 mol of Cl2 reacts with excess Fe?
A)3.1 mol
B)4.6 mol
C)1.5 mol
D)2.3 mol
E)6.9 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
35
What mass of trisodium phosphate is required to prepare 250.0 mL of a solution that is 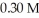
In sodium ion?
A)37 g
B)12 g
C)7.7 g
D)4.1 g
E)3.0 g
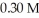
In sodium ion?
A)37 g
B)12 g
C)7.7 g
D)4.1 g
E)3.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
36
For the reaction symbolized as HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)water is both:
A)a reactant and the solute
B)a reactant and the solvent
C)a product and the solute
D)a product and the solvent
E)a liquid and the intermediate
A)a reactant and the solute
B)a reactant and the solvent
C)a product and the solute
D)a product and the solvent
E)a liquid and the intermediate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many mL of 0.024 M solution can be produced from 14.1 mL of 3.0 M solution?
A)(14.1)(0.024)(3.0)mL
B)14.1(0.024/3.0)mL
C)14.1(3.0/0.024)mL
D)(14.1/0.024)/3.0 mL
E)(0.024×3)/14.1 mL
A)(14.1)(0.024)(3.0)mL
B)14.1(0.024/3.0)mL
C)14.1(3.0/0.024)mL
D)(14.1/0.024)/3.0 mL
E)(0.024×3)/14.1 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
38
Gases emitted during volcanic activity often contain high concentrations of hydrogen sulfide and sulfur dioxide.These gases may react to produce deposits of sulfur according to the equation: 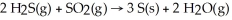
For the complete reaction of 6.41 mol of hydrogen sulfide:
A)308 g of sulfur is formed
B)410 g of sulfur dioxide is consumed
C)231 g of water vapor is produced
D)320 g of total products result
E)628 g of total reactants are consumed
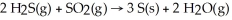
For the complete reaction of 6.41 mol of hydrogen sulfide:
A)308 g of sulfur is formed
B)410 g of sulfur dioxide is consumed
C)231 g of water vapor is produced
D)320 g of total products result
E)628 g of total reactants are consumed

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the molarity of 10.9 g KCl dissolved in 150.0 mL of water?
A)0.0727 M
B)0.146 M
C)0.975 M
D)0.0219 M
E)0.667 M
A)0.0727 M
B)0.146 M
C)0.975 M
D)0.0219 M
E)0.667 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
40
What volume of concentrated acetic acid 
Is needed to prepare 250 mL of a 0.30 M aqueous solution?
A)4.7 mL
B)4.3 mL
C)3.0 mL
D)2.5 mL
E)2.2 mL

Is needed to prepare 250 mL of a 0.30 M aqueous solution?
A)4.7 mL
B)4.3 mL
C)3.0 mL
D)2.5 mL
E)2.2 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
41
Consider the gaseous reaction:
N2H4(g)+ 3 O2(g)→ 2 NO2(g)+ 2 H2O(g)
If the above reaction has a percent yield of 98.5%,what mass in grams of oxygen is needed to produce 49.0 g of NO2(g),assuming an excess of N2H4?
A)50.4 g
B)51.9 g
C)25.9 g
D)23.1 g
E)11.5 g
N2H4(g)+ 3 O2(g)→ 2 NO2(g)+ 2 H2O(g)
If the above reaction has a percent yield of 98.5%,what mass in grams of oxygen is needed to produce 49.0 g of NO2(g),assuming an excess of N2H4?
A)50.4 g
B)51.9 g
C)25.9 g
D)23.1 g
E)11.5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
42
How many grams of CrSO4 will be made from 25.0 grams each of Zn,K2Cr2O7,and H2SO4?
4 Zn + K2Cr2O7 + 7 H2SO4 → 4 ZnSO4 + 2 CrSO4 + K2SO4 + 7 H2O
A)10.8 g
B)28.3 g
C)25.2 g
D)12.6 g
E)37.8 g
4 Zn + K2Cr2O7 + 7 H2SO4 → 4 ZnSO4 + 2 CrSO4 + K2SO4 + 7 H2O
A)10.8 g
B)28.3 g
C)25.2 g
D)12.6 g
E)37.8 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
43
The Haber Process for the production of ammonia is represented by:
3 H2(g)+ N2(g)→ 2 NH3(g)
If a mixture of 30 g of hydrogen with 10 g of nitrogen produced 8.4 g of ammonia,what was the percent yield?
A)84%
B)69%
C)49%
D)28%
E)20%
3 H2(g)+ N2(g)→ 2 NH3(g)
If a mixture of 30 g of hydrogen with 10 g of nitrogen produced 8.4 g of ammonia,what was the percent yield?
A)84%
B)69%
C)49%
D)28%
E)20%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
44
Cryolite is a compound needed for the Hall-Heroult process for producing aluminum.Cryolite is produced by the following reaction:
6 HF + Al(OH)3 + 3 NaOH → Na3AlF6 + 6 H2O
How many grams of cryolite are produced if the reaction has a 94.3% yield and a limiting reagent of 27.8 grams of HF?
A)275 g
B)48.6 g
C)45.9 g
D)15.0 g
E)15.9 g
6 HF + Al(OH)3 + 3 NaOH → Na3AlF6 + 6 H2O
How many grams of cryolite are produced if the reaction has a 94.3% yield and a limiting reagent of 27.8 grams of HF?
A)275 g
B)48.6 g
C)45.9 g
D)15.0 g
E)15.9 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
45
Given the following reactions:
Fe + Br2 → FeBr2
3 FeBr2 + Br2 → Fe3Br8
If each reaction is 82.0% efficient,what mass of iron is necessary to make 8.45 g of Fe3Br8?
A)0.870 g
B)3.73 g
C)2.14 g
D)1.75 g
E)2.61 g
Fe + Br2 → FeBr2
3 FeBr2 + Br2 → Fe3Br8
If each reaction is 82.0% efficient,what mass of iron is necessary to make 8.45 g of Fe3Br8?
A)0.870 g
B)3.73 g
C)2.14 g
D)1.75 g
E)2.61 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many grams of ZnSO4 will be made from 41.0 grams each of Zn,K2Cr2O7,and H2SO4?
4 Zn + K2Cr2O7 + 7 H2SO4 → 4 ZnSO4 + 2 CrSO4 + K2SO4 + 7 H2O
A)67.6 g
B)38.6 g
C)101 g
D)20.6 g
E)82.5 g
4 Zn + K2Cr2O7 + 7 H2SO4 → 4 ZnSO4 + 2 CrSO4 + K2SO4 + 7 H2O
A)67.6 g
B)38.6 g
C)101 g
D)20.6 g
E)82.5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
47
How many grams of a solid mixture containing strontium chloride would one need to make 558 mL of a 0.100 M strontium chloride solution,if the solid mixture contains 58.6% strontium chloride by weight?
A)6.62 g
B)15.1 g
C)8.85 g
D)5.19 g
E)9.52 g
A)6.62 g
B)15.1 g
C)8.85 g
D)5.19 g
E)9.52 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
48
If 0.500 mol of CaCl2 is mixed with 0.200 mol Na3PO4,the maximum amount in moles of Ca3(PO4)2 that can be formed is:
A)0.17
B)0.20
C)0.10
D)0.67
E)0.50
A)0.17
B)0.20
C)0.10
D)0.67
E)0.50

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
49
The Haber Process for the production of ammonia is represented by:
3 H2(g)+ N2(g)→ 2 NH3(g)
If 30 g of hydrogen is mixed with 10 g of nitrogen and the extent of reaction is 0.247,what is the amount of ammonia produced?
A)4.19 g
B)2.47 g
C)7.41 g
D)8.41 g
E)3.70g
3 H2(g)+ N2(g)→ 2 NH3(g)
If 30 g of hydrogen is mixed with 10 g of nitrogen and the extent of reaction is 0.247,what is the amount of ammonia produced?
A)4.19 g
B)2.47 g
C)7.41 g
D)8.41 g
E)3.70g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
Al + Fe2O3 → Al2O3 + Fe
A)4
B)6
C)12
D)9
E)8
Al + Fe2O3 → Al2O3 + Fe
A)4
B)6
C)12
D)9
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
51
Given the reaction:
P4(l)+ 6 Cl2(g)→ 4 PCl3(l)
If the percent yield is 82%,what mass of P4 is required to obtain 2.30 g PCl3 (Cl2 in excess)?
A)0.63 g
B)0.52 g
C)0.43 g
D)0.16 g
E)0.95 g
P4(l)+ 6 Cl2(g)→ 4 PCl3(l)
If the percent yield is 82%,what mass of P4 is required to obtain 2.30 g PCl3 (Cl2 in excess)?
A)0.63 g
B)0.52 g
C)0.43 g
D)0.16 g
E)0.95 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
CaC2(s)+ H2O(l)→ Ca(OH)2(s)+ C2H2(g)
A)8
B)4
C)5
D)10
E)9
CaC2(s)+ H2O(l)→ Ca(OH)2(s)+ C2H2(g)
A)8
B)4
C)5
D)10
E)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
KClO3(s)→ KCl(s)+ O2(g)
A)11
B)10
C)5
D)7
E)6
KClO3(s)→ KCl(s)+ O2(g)
A)11
B)10
C)5
D)7
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
54
One source of iodine is sodium iodate.Iodine is produced by a series of reactions.The first reaction is a reduction reaction with sodium hydrogen sulfite.
IO3-( aq)+ 3 HSO3- (aq)→ I- (aq)+ 3 SO42- (aq)+ 3 H+(aq)
5 I- (aq)+ IO3- (aq)+ 6 H+ (aq)→ 3 I2(s)+ 3 H2O
How many grams of iodine are produced from 1.00 × 102 grams of NaHSO3 if each reaction has a 95.0% yield?
A)48.8 g
B)51.4 g
C)185 g
D)46.3 g
E)44.0 g
IO3-( aq)+ 3 HSO3- (aq)→ I- (aq)+ 3 SO42- (aq)+ 3 H+(aq)
5 I- (aq)+ IO3- (aq)+ 6 H+ (aq)→ 3 I2(s)+ 3 H2O
How many grams of iodine are produced from 1.00 × 102 grams of NaHSO3 if each reaction has a 95.0% yield?
A)48.8 g
B)51.4 g
C)185 g
D)46.3 g
E)44.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
55
42.6 g Cu are combined with 84.0 g of HNO3 according to the reaction:
3 Cu + 8 HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O
Which reagent is limiting and how many grams of Cu(NO3)2 are produced?
A)Cu,93.8 g
B)HNO3,93.8 g
C)Cu,125.6 g
D)HNO3,125.6 g
E)Cu(NO3)2,125.6 g
3 Cu + 8 HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O
Which reagent is limiting and how many grams of Cu(NO3)2 are produced?
A)Cu,93.8 g
B)HNO3,93.8 g
C)Cu,125.6 g
D)HNO3,125.6 g
E)Cu(NO3)2,125.6 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
56
For the reaction 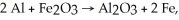
The stoichiometric number for Al2O3 is:
A)+2
B)+1
C)-1
D)-2
E)0
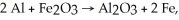
The stoichiometric number for Al2O3 is:
A)+2
B)+1
C)-1
D)-2
E)0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
57
Given the reaction: 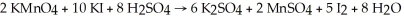
How many moles of K2SO4 are produced by allowing five moles each of KMnO4,KI,and H2SO4 to react?
A)3 mol
B)1 mol
C)2 mol
D)4 mol
E)5 mol
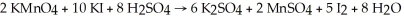
How many moles of K2SO4 are produced by allowing five moles each of KMnO4,KI,and H2SO4 to react?
A)3 mol
B)1 mol
C)2 mol
D)4 mol
E)5 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
58
If 8.52 g each of zinc,potassium dichromate,and sulfuric acid are reacted by the reaction:
4 Zn + K2Cr2O7 + 7 H2SO4→ 4 ZnSO4 + 2 CrSO4 +K2SO4 + 7 H2O
How many grams of potassium dichromate will be left unreacted?
A)1.05 g
B)2.84 g
C)7.30 g
D)4.87 g
E)3.65 g
4 Zn + K2Cr2O7 + 7 H2SO4→ 4 ZnSO4 + 2 CrSO4 +K2SO4 + 7 H2O
How many grams of potassium dichromate will be left unreacted?
A)1.05 g
B)2.84 g
C)7.30 g
D)4.87 g
E)3.65 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
59
If 0.500 mol of CaCl2 is mixed with 0.200 mol Na3PO4,the maximum amount in moles of Ca3(PO4)2 that can be formed is 0.10 moles.Calculate the extent of reaction ,x.
A)0.2
B)0.10
C)0.35
D)0
E)0.65
A)0.2
B)0.10
C)0.35
D)0
E)0.65

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
60
For the reaction 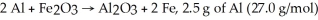
And 7.2 g of Fe2O3 (159.8 g/mol)produce 5.03 g of Fe (55.9 g/mol).Calculate the extent of reaction ,x.
A)0.362
B)0.089
C)0.045
D)0.092
E)0.405
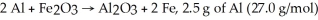
And 7.2 g of Fe2O3 (159.8 g/mol)produce 5.03 g of Fe (55.9 g/mol).Calculate the extent of reaction ,x.
A)0.362
B)0.089
C)0.045
D)0.092
E)0.405

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
61
"Washing soda" (sodium carbonate)may be used to "soften" water by the removal of certain ions that would otherwise react with common soaps.When the "hardness" is due to calcium ion,the "softening" process may be represented as:
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
What mass of sodium carbonate would be required to remove essentially all of the calcium ion from 750 L of solution containing 43 mg Ca2+ per liter?
A)85 g
B)67 g
C)12 g
D)48 g
E)22 g
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
What mass of sodium carbonate would be required to remove essentially all of the calcium ion from 750 L of solution containing 43 mg Ca2+ per liter?
A)85 g
B)67 g
C)12 g
D)48 g
E)22 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
62
If an aqueous solution containing 46 g of sodium carbonate per liter is mixed with an equal volume of 0.20 M aqueous hydrochloric acid,what would be the molarity of sodium chloride in the final solution,assuming volumes were additive?
A)0.10 M
B)0.20 M
C)0.22 M
D)0.43 M
E)0.50 M
A)0.10 M
B)0.20 M
C)0.22 M
D)0.43 M
E)0.50 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the molarity of a sucrose solution (C12H22O11)if 110.0 g of a 92.0% pure solid is dissolved per 250.0 mL of water?
A)0.296 M
B)1.29 M
C)0.321 M
D)1.40 M
E)1.18 M
A)0.296 M
B)1.29 M
C)0.321 M
D)1.40 M
E)1.18 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
64
Write the complete balanced equation for the complete combustion reaction expected to occur between C3H7OH and O2.
A)C3H7OH + O2 → 3 CO2 + 4 H2O
B)3 C3H7OH + 9 O2 → 6 CO2 + 8 H2O + 3 C
C)2 C3H7OH + 6 O2 → CO + 8 H2O
D)2 C3H7OH + 9 O2 → 6 CO2 + 8 H2O
E)2 C3H7OH + 9 O2 → 6 C + 8 H2 + 10 O2
A)C3H7OH + O2 → 3 CO2 + 4 H2O
B)3 C3H7OH + 9 O2 → 6 CO2 + 8 H2O + 3 C
C)2 C3H7OH + 6 O2 → CO + 8 H2O
D)2 C3H7OH + 9 O2 → 6 CO2 + 8 H2O
E)2 C3H7OH + 9 O2 → 6 C + 8 H2 + 10 O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the molarity of formaldehyde in a solution containing 0.25 g of formaldehyde (CH2O)per mL?
A)2.5 M
B)25 M
C)8.3 M
D)83 M
E)4.0 M
A)2.5 M
B)25 M
C)8.3 M
D)83 M
E)4.0 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
H2(g)+ O2(g)→ H2O(l)
A)4
B)5
C)8
D)9
E)7
H2(g)+ O2(g)→ H2O(l)
A)4
B)5
C)8
D)9
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
67
The chemical reaction occurring during the discharge of a lead storage battery can be represented by the equation: 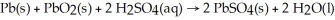
What mass of lead sulfate would result from the complete reaction of 41.4 g of lead?
A)57.6 g
B)60.5 g
C)105 g
D)115 g
E)121 g
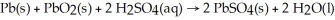
What mass of lead sulfate would result from the complete reaction of 41.4 g of lead?
A)57.6 g
B)60.5 g
C)105 g
D)115 g
E)121 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the molarity of methanol, 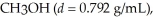
If 150.0 mL is dissolved in enough water to make 4.00 L of solution?
A)3.71 M
B)1.17 M
C)1.48 M
D)0.927 M
E)0.734 M
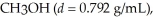
If 150.0 mL is dissolved in enough water to make 4.00 L of solution?
A)3.71 M
B)1.17 M
C)1.48 M
D)0.927 M
E)0.734 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
69
Our task is to measure the volume of blood in an elephant.One way to do this would be to drain its blood into a suitable container.Aside from harmful side effects which this method has on the elephant,it will not be successful,as blood will still remain behind in the tissues.Hence,we will inject 2.00 ml of a 2.00 M solution of a dye which the elephant will not appreciably metabolize or excrete in one hour and then measure the concentration of this dye in the bloodstream (our sample being taken from another leg than the point of injection)after 30 minutes,a sufficient time to thoroughly mix the dye in the bloodstream.The concentration of dye at this point is 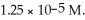
What is the volume of blood in the elephant?
A)320 L
B)320000 L
C)3.2 L
D)80 L
E)800 L
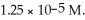
What is the volume of blood in the elephant?
A)320 L
B)320000 L
C)3.2 L
D)80 L
E)800 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
CH3OH → CO + H2
A)3
B)8
C)5
D)4
E)7
CH3OH → CO + H2
A)3
B)8
C)5
D)4
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
71
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
Al(s)+ HCl(aq)→ AlCl3(aq)+ H2(g)
A)13
B)11
C)18
D)19
E)21
Al(s)+ HCl(aq)→ AlCl3(aq)+ H2(g)
A)13
B)11
C)18
D)19
E)21

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
C2H6 + O2 → CO2 + H2O
A)5
B)10
C)19
D)21
E)25
C2H6 + O2 → CO2 + H2O
A)5
B)10
C)19
D)21
E)25

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
73
You have 10.00 L of a 0.350 M KCl solution,but you need a solution that is 0.450 M.What volume of water,in L,would you evaporate from the solution?
A)4.38 L
B)3.50 L
C)2.85 L
D)7.77 L
E)2.22 L
A)4.38 L
B)3.50 L
C)2.85 L
D)7.77 L
E)2.22 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
74
What mass of MgCl2 in grams must be added to 250.0 mL of a 0.25 M MgCl2 solution to produce a 0.40 M solution,assuming no change of volume upon addition?
A)9.5 g
B)6.0 g
C)2.2 g
D)3.6 g
E)19 g
A)9.5 g
B)6.0 g
C)2.2 g
D)3.6 g
E)19 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the sum of the coefficients when the following equation is balanced with the smallest integer coefficients?
H2SO3 + Al(OH)3 → Al2(SO3)3 + H2O
A)4
B)5
C)11
D)12
E)14
H2SO3 + Al(OH)3 → Al2(SO3)3 + H2O
A)4
B)5
C)11
D)12
E)14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
76
What mass of water is produced in the metathesis reaction 75.2 g Zn(OH)2 with 43.4 g HCl?
A)13.6 g
B)27.3 g
C)10.7 g
D)21.4 g
E)31.8 g
A)13.6 g
B)27.3 g
C)10.7 g
D)21.4 g
E)31.8 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
77
The molarity of a solution that contains 14.7 g of H2SO4 in 200.0 mL solution is ________.
A)1.5 M
B)0.75 M
C)0.77 M
D)7.4 M
E)3.0 M
A)1.5 M
B)0.75 M
C)0.77 M
D)7.4 M
E)3.0 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
78
How much Cl2,in g,is required to produce 12.0 g CCl4 according to the following reaction? 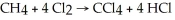
A)12.0 g
B)5.52 g
C)22.1 g
D)1.38 g
E)11.0 g
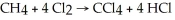
A)12.0 g
B)5.52 g
C)22.1 g
D)1.38 g
E)11.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
79
What mass of oxygen gas would be consumed by the complete combustion of 7.5 g of a mixture of propane (C3H8)and butane (C4H10)in the mole ratio of 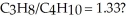
A)3.9 g
B)11 g
C)14 g
D)21 g
E)27 g
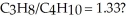
A)3.9 g
B)11 g
C)14 g
D)21 g
E)27 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck
80
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
PCl3(l)+ Cl2(g)+ P4O10(s)→ POCl3(l)
A)3
B)18
C)45
D)10
E)23
PCl3(l)+ Cl2(g)+ P4O10(s)→ POCl3(l)
A)3
B)18
C)45
D)10
E)23

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 170 في هذه المجموعة.
فتح الحزمة
k this deck








