Deck 3: Radiation From the Sun
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/50
العب
ملء الشاشة (f)
Deck 3: Radiation From the Sun
1
The Montreal protocol is a
A)treaty to protect against global warming.
B)treaty to reduce the amount of CFCs produced in the world.
C)list of substitutes for CFCs.
D)way to destroy CFCs in the stratosphere.
A)treaty to protect against global warming.
B)treaty to reduce the amount of CFCs produced in the world.
C)list of substitutes for CFCs.
D)way to destroy CFCs in the stratosphere.
treaty to reduce the amount of CFCs produced in the world.
2
Which is correct?
A)Ozone forms by combining an oxygen atom with an oxygen molecule
B)There is a dynamic steady state of ozone in the stratosphere
C)UV radiation will dissociate ozone into an oxygen atom and an oxygen molecule
D)All of these choices are correct
A)Ozone forms by combining an oxygen atom with an oxygen molecule
B)There is a dynamic steady state of ozone in the stratosphere
C)UV radiation will dissociate ozone into an oxygen atom and an oxygen molecule
D)All of these choices are correct
All of these choices are correct
3
As the ozone hole gets more pronounced,with time,one expects the incidence of skin cancer to
A)decrease worldwide.
B)increase worldwide.
C)increase in the northern hemisphere and decrease in the southern hemisphere.
D)decrease in the northern hemisphere and decrease in the northern hemisphere.
A)decrease worldwide.
B)increase worldwide.
C)increase in the northern hemisphere and decrease in the southern hemisphere.
D)decrease in the northern hemisphere and decrease in the northern hemisphere.
increase worldwide.
4
Wavelength is the
A)number of waves passing a fixed point in one second.
B)height of the wave.
C)distance between successive peaks in a wave.
D)distance between a peak of one wave and the next trough.
A)number of waves passing a fixed point in one second.
B)height of the wave.
C)distance between successive peaks in a wave.
D)distance between a peak of one wave and the next trough.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
5
When it reaches its largest size,the ozone hole over the Antarctic is
A)about as large as North America.
B)about the same size as France.
C)smaller than Iceland.
D)about the same size as Canada.
A)about as large as North America.
B)about the same size as France.
C)smaller than Iceland.
D)about the same size as Canada.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which is the correct Lewis structure?
A)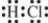
B)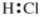
C)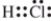
D)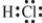
A)
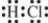
B)
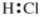
C)
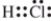
D)
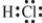

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
7
Increasing wavelength of light goes in this order.
A)Ultraviolet > visible > infrared
B)Visible > infrared > ultraviolet
C)Infrared > visible > ultraviolet
D)Ultraviolet > infrared > visible
A)Ultraviolet > visible > infrared
B)Visible > infrared > ultraviolet
C)Infrared > visible > ultraviolet
D)Ultraviolet > infrared > visible

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
8
During the Antarctic spring,ozone is destroyed at a greater rate than it is formed
A)on the surface of atmospheric ice crystals.
B)in a process that is catalytic.
C)in polar stratospheric clouds.
D)All of these choices are correct
A)on the surface of atmospheric ice crystals.
B)in a process that is catalytic.
C)in polar stratospheric clouds.
D)All of these choices are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
9
The speed of light in air
A)depends only on the frequency of the light.
B)depends only on the wavelength of light.
C)is independent of the wavelength and frequency of light.
D)depends on both the wavelength and the frequency of light.
A)depends only on the frequency of the light.
B)depends only on the wavelength of light.
C)is independent of the wavelength and frequency of light.
D)depends on both the wavelength and the frequency of light.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
10
When only one pair of shared electrons is involved in a covalent bond,the linkage is called a bond.
A)triple
B)single
C)double
D)resonant
A)triple
B)single
C)double
D)resonant

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
11
Ozone in our atmosphere is important because it
A)absorbs some UV radiation.
B)helps trees grow.
C)reacts with excess CO2.
D)reflects IR radiation.
A)absorbs some UV radiation.
B)helps trees grow.
C)reacts with excess CO2.
D)reflects IR radiation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which statement is correct?
A)UV-A is the most energetic of the three forms of UV light
B)UV-B is the most energetic of the three forms of UV light
C)UV-C is the most energetic of the three forms of UV light
D)UV-A,UV-B,and UV-C are equally energetic
A)UV-A is the most energetic of the three forms of UV light
B)UV-B is the most energetic of the three forms of UV light
C)UV-C is the most energetic of the three forms of UV light
D)UV-A,UV-B,and UV-C are equally energetic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which contributes to the ozone hole?
A)Automobile exhaust
B)Chlorofluorocarbons (CFCs)
C)Loss of northern forests
D)All of these choices are correct
A)Automobile exhaust
B)Chlorofluorocarbons (CFCs)
C)Loss of northern forests
D)All of these choices are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
14
HFCs may be used to replace CFCs.Which compound is a HFC?
A)CH2Cl-CCl2F
B)CH2FCl
C)CF3CH2F
D)CHClF2
A)CH2Cl-CCl2F
B)CH2FCl
C)CF3CH2F
D)CHClF2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
15
In Earth's atmosphere,where is the ozone layer? 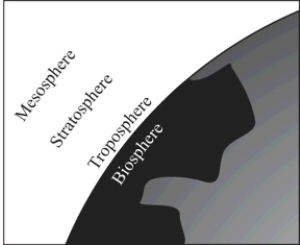
A)Troposphere
B)Biosphere
C)Mesosphere
D)Stratosphere

A)Troposphere
B)Biosphere
C)Mesosphere
D)Stratosphere

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
16
DNA,the genetic material of living organisms,is damaged by light in the
A)visible region of the spectrum.
B)ultraviolet region,especially below a wavelength of 320 nm.
C)ultraviolet region,especially above a wavelength of 340 nm.
D)infrared region of the spectrum.
A)visible region of the spectrum.
B)ultraviolet region,especially below a wavelength of 320 nm.
C)ultraviolet region,especially above a wavelength of 340 nm.
D)infrared region of the spectrum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which color in the rainbow has the shortest wavelength?
A)Orange
B)Red
C)Yellow
D)Blue
A)Orange
B)Red
C)Yellow
D)Blue

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
18
The ozone hole is most prominent on the Earth over
A)North America.
B)Europe.
C)Africa.
D)Antarctica.
A)North America.
B)Europe.
C)Africa.
D)Antarctica.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the relationship between stratospheric levels of atomic chlorine and ozone?
A)As chlorine increases,ozone increases
B)As chlorine increases,ozone decreases
C)As chlorine changes,the effect on the ozone level is unpredictable
D)As chlorine changes,there is no effect of the ozone level
A)As chlorine increases,ozone increases
B)As chlorine increases,ozone decreases
C)As chlorine changes,the effect on the ozone level is unpredictable
D)As chlorine changes,there is no effect of the ozone level

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
20
The structure of ozone most closely resembles a
A)linear molecule with different lengths of chemical bonds,for example,
B)linear molecule with the same length of chemical bonds,for example,
C)bent molecule with different lengths of chemical bonds,for example,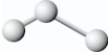
D)bent molecule with the same length of chemical bonds,for example,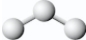
A)linear molecule with different lengths of chemical bonds,for example,

B)linear molecule with the same length of chemical bonds,for example,

C)bent molecule with different lengths of chemical bonds,for example,

D)bent molecule with the same length of chemical bonds,for example,


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
21
UV-B radiation has a frequency of approximately 1017 s¯1.What is the energy of a photon of this light?
A)1.99 × 10¯42 J
B)6.63 × 10¯17 J
C)4.19 × 108 J
D)1.51 × 1050 J
A)1.99 × 10¯42 J
B)6.63 × 10¯17 J
C)4.19 × 108 J
D)1.51 × 1050 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
22
Chlorofluorocarbons rise to the stratosphere and
A)react directly with stratospheric ozone to destroy it.
B)interact with UV energy to produce free radicals that destroy ozone.
C)interact with UV energy to produce free radicals that react with oxygen to create ozone.
D)react with free radicals to remove carbon dioxide.
A)react directly with stratospheric ozone to destroy it.
B)interact with UV energy to produce free radicals that destroy ozone.
C)interact with UV energy to produce free radicals that react with oxygen to create ozone.
D)react with free radicals to remove carbon dioxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
23
Light behaves like
A)a particle.
B)a wave.
C)both a particle and a wave.
D)neither a particle nor a wave.
A)a particle.
B)a wave.
C)both a particle and a wave.
D)neither a particle nor a wave.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
24
Halons differ from CFCs in that the atoms of ________ replace some ________ atoms.
A)iodine;chlorine
B)hydrogen;chlorine
C)bromine;chlorine
D)silicon;carbon
A)iodine;chlorine
B)hydrogen;chlorine
C)bromine;chlorine
D)silicon;carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
25
The "ozone layer" is found
A)only around the equator.
B)in the troposphere.
C)in the stratosphere.
D)in the mesosphere.
A)only around the equator.
B)in the troposphere.
C)in the stratosphere.
D)in the mesosphere.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which product of the ultraviolet decomposition of CFCs acts as the catalyst for ozone decomposition?
A)Oxygen atoms
B)Chlorine atoms
C)Fluorine atoms
D)Hydrogen atoms
A)Oxygen atoms
B)Chlorine atoms
C)Fluorine atoms
D)Hydrogen atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which is one of the Lewis structures for ozone?
A)
B)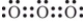
C)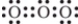
D)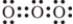
A)

B)
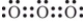
C)
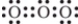
D)
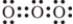

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
28
WUKF FM transmits at 93.5 MHz.What is the wavelength of the electromagnetic radiation that carries the station's signal?
A)6.42 × 10¯9 m
B)3.21 m
C)3.21 × 106 m
D)3.12 × 1015 m
A)6.42 × 10¯9 m
B)3.21 m
C)3.21 × 106 m
D)3.12 × 1015 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
29
HCFCs have been developed to replace CFCs as refrigerants.Which property of these new compounds makes them environmentally superior to CFCs?
A)Greater reactivity leads to decomposition at elevations below the stratospheric ozone concentration maximum
B)Lower reactivity makes them stable even in the intense ultraviolet light in the stratosphere
C)Their higher molecular weight prevents them from reaching the stratosphere
D)They do not contain chlorine
A)Greater reactivity leads to decomposition at elevations below the stratospheric ozone concentration maximum
B)Lower reactivity makes them stable even in the intense ultraviolet light in the stratosphere
C)Their higher molecular weight prevents them from reaching the stratosphere
D)They do not contain chlorine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
30
The two chemical bonds and geometry of water are best represented by
A)
B)
C)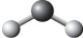
D)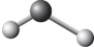
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which Lewis structure for formaldehyde (CH2O) is correct? 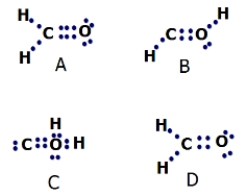
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
32
Why are HFCs environmentally superior to the currently used HCFCs?
A)HFCs are not flammable
B)HFCs do not contain chlorine
C)HFCs are lighter and may be transported more easily
D)HFCs are less reactive than HCFCs
A)HFCs are not flammable
B)HFCs do not contain chlorine
C)HFCs are lighter and may be transported more easily
D)HFCs are less reactive than HCFCs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
33
Free radicals are
A)highly reactive chemical species.
B)species with unpaired electrons.
C)species such as H• and •OH.
D)All of these correctly describe free radicals
A)highly reactive chemical species.
B)species with unpaired electrons.
C)species such as H• and •OH.
D)All of these correctly describe free radicals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which region of the ultraviolet spectrum is absorbed least by the atmosphere?
A)UV-A
B)UV-B
C)UV-C
D)They are all absorbed approximately equally
A)UV-A
B)UV-B
C)UV-C
D)They are all absorbed approximately equally

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
35
In reference to waves,frequency is the
A)number of waves passing a fixed point in one second.
B)height of the wave.
C)distance between successive peaks in a wave.
D)distance between a peak in a wave to the next trough.
A)number of waves passing a fixed point in one second.
B)height of the wave.
C)distance between successive peaks in a wave.
D)distance between a peak in a wave to the next trough.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
36
CFCs were originally developed to replace which refrigerant compound(s)?
A)Ice
B)HCFCs
C)Ammonia and sulfur dioxide
D)Propane
A)Ice
B)HCFCs
C)Ammonia and sulfur dioxide
D)Propane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
37
Yellow light has a wavelength of 580 nm.What is the frequency of this light?
A)2.39 × 10¯19 s¯1
B)1.80 × 10¯7 s¯1
C)5.17 × 105 s¯1
D)5.17 × 1014 s¯1
A)2.39 × 10¯19 s¯1
B)1.80 × 10¯7 s¯1
C)5.17 × 105 s¯1
D)5.17 × 1014 s¯1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
38
HCFCs are a temporary solution to the problem of ozone depletion and will be replaced over the next 20 years by which class of compounds?
A)HFCs
B)CFCs
C)Halons
D)HFBCs
A)HFCs
B)CFCs
C)Halons
D)HFBCs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
39
Single bonds,double bonds,and triple bonds
A)have 1,2,and 3 shared electrons,respectively.
B)have 2,4,and 6 shared electrons,respectively.
C)have 3,6,and 9 shared electrons,respectively.
D)are only possible between carbon atoms.
A)have 1,2,and 3 shared electrons,respectively.
B)have 2,4,and 6 shared electrons,respectively.
C)have 3,6,and 9 shared electrons,respectively.
D)are only possible between carbon atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
40
Decreased stratospheric ozone concentrations may lead to
A)increased incidences of melanomas.
B)harm to young marine life.
C)an increased occurrence of cataracts.
D)All of these choices are correct
A)increased incidences of melanomas.
B)harm to young marine life.
C)an increased occurrence of cataracts.
D)All of these choices are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
41
Why are HFCs inappropriate for long-term replacement of CFCs?
A)They are flammable
B)They are very toxic
C)They absorb infrared radiation
D)They are an appropriate replacement
A)They are flammable
B)They are very toxic
C)They absorb infrared radiation
D)They are an appropriate replacement

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
42
The morning newspaper reports a UV Index Forecast of 6.5.What precautions,if any should a fair skinned person take?
A)None
B)Only sunglasses and maybe a hat is enough
C)Reduce exposure between 10 a.m.and 4 p.m.in addition to SPF 15+ sunscreen
D)All precautions must be taken;this is an extreme UV day
A)None
B)Only sunglasses and maybe a hat is enough
C)Reduce exposure between 10 a.m.and 4 p.m.in addition to SPF 15+ sunscreen
D)All precautions must be taken;this is an extreme UV day

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
43
How do mineral nanoparticles in sunscreens protect from UV radiation?
A)The particles scatter incoming UV light
B)The particles absorb incoming UV light
C)The particles dissolve with incoming UV light
D)None of these choices are correct
A)The particles scatter incoming UV light
B)The particles absorb incoming UV light
C)The particles dissolve with incoming UV light
D)None of these choices are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
44
The O2 molecule breaks apart at lower wavelengths than the O3 molecule.What is the main reason for this? (Hint: Draw the Lewis structures. )
A)O2 is more reactive than O3
B)O3 is more reactive than O2
C)The average bond in O3 is shorter and stronger than that of O2
D)The average bond in O2 is shorter and stronger than that of O3
A)O2 is more reactive than O3
B)O3 is more reactive than O2
C)The average bond in O3 is shorter and stronger than that of O2
D)The average bond in O2 is shorter and stronger than that of O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which compounds are used in mineral-based sunscreens?
A)ZnO and TiO2
B)ZnO and CdS
C)TiO2 and SiO2
D)CdS and SiO2
A)ZnO and TiO2
B)ZnO and CdS
C)TiO2 and SiO2
D)CdS and SiO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the role of polar stratospheric clouds (PSCs) on the destruction of ozone?
A)The cold clouds react with ozone to make oxygen molecules and oxygen atoms
B)Chemical reactions occur on the clouds that convert molecules that do no damage to those that deplete ozone
C)They play no role
D)The clouds are made of chlorine atoms from CFCs
A)The cold clouds react with ozone to make oxygen molecules and oxygen atoms
B)Chemical reactions occur on the clouds that convert molecules that do no damage to those that deplete ozone
C)They play no role
D)The clouds are made of chlorine atoms from CFCs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the following is most biologically damaging type of radiation?
A)UV-A
B)UV-B
C)UV-C
D)Infrared
A)UV-A
B)UV-B
C)UV-C
D)Infrared

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following compounds is useful for putting out fires and does not deplete stratospheric ozone concentrations?
A)Halon-1211
B)CFC-113
C)HFCs
D)Methyl Bromide
A)Halon-1211
B)CFC-113
C)HFCs
D)Methyl Bromide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is special about the South Pole versus the North Pole that leads to ozone depletion only at the south Pole?
A)Ozone molecules are broken up by magnetic forces at the South Pole
B)The atmosphere is colder at the North Pole than at the South Pole
C)Polar stratospheric clouds form almost exclusively at the South Pole
D)There is more land mass at the South Pole than at the North Pole
A)Ozone molecules are broken up by magnetic forces at the South Pole
B)The atmosphere is colder at the North Pole than at the South Pole
C)Polar stratospheric clouds form almost exclusively at the South Pole
D)There is more land mass at the South Pole than at the North Pole

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
50
Arrange these types of radiation in order of increasing energy per photon: gamma rays,infrared radiation,radio waves,visible light,UV (ultra violet).
A)Radio waves < visible light < UV < infrared radiation < gamma rays
B)Radio waves < infrared radiation < visible light < UV < gamma rays
C)Infrared radiation < radio waves < visible light < UV < gamma rays
D)Gamma rays < infrared radiation < UV < radio waves < visible light
A)Radio waves < visible light < UV < infrared radiation < gamma rays
B)Radio waves < infrared radiation < visible light < UV < gamma rays
C)Infrared radiation < radio waves < visible light < UV < gamma rays
D)Gamma rays < infrared radiation < UV < radio waves < visible light

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck








