Deck 8: Chemical Reactions and Chemical Quantities
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/79
العب
ملء الشاشة (f)
Deck 8: Chemical Reactions and Chemical Quantities
1
Determine the limiting reactant (LR)and the mass (in g)of nitrogen that can be formed from 50.0 g N2O4 and 45.0 g N2H4.Some possibly useful molar masses are as follows: N2O4 = 92.02 g/mol,N2H4 = 32.05 g/mol. N2O4(l)+ 2 N2H4(l)→ 3 N2(g)+ 4 H2O(g)
A) LR = N2H4, 59.0 g N2 formed
B) LR = N2O4, 105 g N2 formed
C) LR = N2O4, 45.7 g N2 formed
D) LR = N2H4, 13.3 g N2 formed
E) No LR, 45.0 g N2 formed
A) LR = N2H4, 59.0 g N2 formed
B) LR = N2O4, 105 g N2 formed
C) LR = N2O4, 45.7 g N2 formed
D) LR = N2H4, 13.3 g N2 formed
E) No LR, 45.0 g N2 formed
LR = N2O4, 45.7 g N2 formed
2
Give the theoretical yield,in moles,of CO2 from the reaction of 4.00 moles of C8H18 with 4.00 moles of O2. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
A) 0.640 moles
B) 64.0 moles
C) 2.56 moles
D) 16.0 moles
A) 0.640 moles
B) 64.0 moles
C) 2.56 moles
D) 16.0 moles
2.56 moles
3
Consider the following reaction.How many moles of oxygen are required to produce 2.33 moles of water? Assume that there is excess C3H7SH present.
C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
A) 1.55 moles O2
B) 3.50 moles O2
C) 2.33 moles O2
D) 4.14 moles O2
E) 6.21 moles O2
C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
A) 1.55 moles O2
B) 3.50 moles O2
C) 2.33 moles O2
D) 4.14 moles O2
E) 6.21 moles O2
3.50 moles O2
4
Consider the following balanced reaction.What mass (in g)of CO2 can be formed from 288 mg of O2? Assume that there is excess C3H7SH present.
C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
A) 0.396 g CO2
B) 0.209 g CO2
C) 0.792 g CO2
D) 0.126 g CO2
E) 0.198 g CO2
C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
A) 0.396 g CO2
B) 0.209 g CO2
C) 0.792 g CO2
D) 0.126 g CO2
E) 0.198 g CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
5
A physical change
A) occurs when iron rusts.
B) occurs when sugar is heated into caramel.
C) occurs when glucose is converted into energy within your cells.
D) occurs when water is evaporated.
E) occurs when propane is burned for heat.
A) occurs when iron rusts.
B) occurs when sugar is heated into caramel.
C) occurs when glucose is converted into energy within your cells.
D) occurs when water is evaporated.
E) occurs when propane is burned for heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
6
According to the following reaction,how many grams of sulfur are formed when 37.4 g of water are formed?
2 H2S(g)+ SO2(g)→ 3 S(s)+ 2H2O(l)
A) 99.8 g S
B) 66.6 g S
C) 56.1 g S
D) 44.4 g S
E) 14.0 g S
2 H2S(g)+ SO2(g)→ 3 S(s)+ 2H2O(l)
A) 99.8 g S
B) 66.6 g S
C) 56.1 g S
D) 44.4 g S
E) 14.0 g S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
7
How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol.
4 KNO3(s)→ 2 K2O(s)+ 2 N2(g)+ 5 O2(g)
A) 0.290 mol O2
B) 0.580 mol O2
C) 18.5 mol O2
D) 0.724 mol O2
E) 1.73 mol O2
4 KNO3(s)→ 2 K2O(s)+ 2 N2(g)+ 5 O2(g)
A) 0.290 mol O2
B) 0.580 mol O2
C) 18.5 mol O2
D) 0.724 mol O2
E) 1.73 mol O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
8
A 12.39 g sample of phosphorus reacts with 42.54 g of chlorine to form only phosphorus trichloride (PCl3).If it is the only product,what mass of PCl3 is formed?
A) 30.15 g
B) 54.93 g
C) 140.01 g
D) 79.71 g
E) 91.86 g
A) 30.15 g
B) 54.93 g
C) 140.01 g
D) 79.71 g
E) 91.86 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
9
Two samples of potassium iodide are decomposed into their constituent elements.The first sample produced 13.0 g of potassium and 42.3 g of iodine.If the second sample produced 24.4 kg of potassium,how many kg of iodine were produced?
A) 13.3 kg
B) 22.5 kg
C) 79.4 kg
D) 44.4 kg
E) 92.4 kg
A) 13.3 kg
B) 22.5 kg
C) 79.4 kg
D) 44.4 kg
E) 92.4 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
10
Determine the theoretical yield of HCl if 60.0 g of BCl3 and 37.5 g of H2O are reacted according to the following balanced reaction.A possibly useful molar mass is BCl3 = 117.16 g/mol. BCl3(g)+ 3 H2O(l)→ H3BO3(s)+ 3 HCl(g)
A) 75.9 g HCl
B) 132 g HCl
C) 187 g HCl
D) 56.0 g HCl
E) 25.3 g HCl
A) 75.9 g HCl
B) 132 g HCl
C) 187 g HCl
D) 56.0 g HCl
E) 25.3 g HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following represents a physical property?
A) Sodium metal is extremely reactive with chlorine gas.
B) Mercury is a silvery liquid at room temperature.
C) Aluminum has a tendency to "rust."
D) Butane is highly flammable.
E) Argon has an unreactive nature.
A) Sodium metal is extremely reactive with chlorine gas.
B) Mercury is a silvery liquid at room temperature.
C) Aluminum has a tendency to "rust."
D) Butane is highly flammable.
E) Argon has an unreactive nature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
12
Two samples of calcium fluoride are decomposed into their constituent elements.The first sample produced 0.154 g of calcium and 0.146 g of fluorine.If the second sample produced 294 mg of fluorine,how many g of calcium were formed?
A) 0.280 g
B) 3.09 × 102 g
C) 3.13 g
D) 0.309 g
E) 2.80 × 102 g
A) 0.280 g
B) 3.09 × 102 g
C) 3.13 g
D) 0.309 g
E) 2.80 × 102 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following represents a chemical property of hydrogen gas?
A) It is gaseous at room temperature.
B) It is less dense than air.
C) It reacts explosively with oxygen.
D) It is colorless.
E) It is tasteless.
A) It is gaseous at room temperature.
B) It is less dense than air.
C) It reacts explosively with oxygen.
D) It is colorless.
E) It is tasteless.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
14
Global warming is thought to be caused by the increase of one particular gas.Name the gas.
A) oxygen
B) carbon monoxide
C) carbon dioxide
D) nitrogen
E) helium
A) oxygen
B) carbon monoxide
C) carbon dioxide
D) nitrogen
E) helium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
15
A 14.01 g sample of N2 reacts with 3.02 g of H2 to form ammonia (NH3).If ammonia is the only product,what mass of ammonia is formed?
A) 17.03 g
B) 1.10 g
C) 14.01 g
D) 3.02 g
E) 23.07 g
A) 17.03 g
B) 1.10 g
C) 14.01 g
D) 3.02 g
E) 23.07 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
16
Consider the following balanced reaction.How many grams of water are required to form 75.9 g of HNO3? Assume that there is excess NO2 present.The molar masses are as follows: H2O = 18.02 g/mol,HNO3 = 63.02 g/mol.
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A) 38.0 g H2O
B) 21.7 g H2O
C) 43.4 g H2O
D) 10.9 g H2O
E) 26.5 g H2O
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A) 38.0 g H2O
B) 21.7 g H2O
C) 43.4 g H2O
D) 10.9 g H2O
E) 26.5 g H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
17
How many molecules of H2S are required to form 79.0 g of sulfur according to the following reaction? Assume excess SO2.
2 H2S(g)+ SO2(g)→ 3 S(s)+ 2H2O(l)
A) 1.48 × 1024 molecules H2S
B) 9.89 × 1023 molecules H2S
C) 5.06 × 1025 molecules H2S
D) 3.17 × 1025 molecules H2S
E) 2.44 × 1023 molecules H2S
2 H2S(g)+ SO2(g)→ 3 S(s)+ 2H2O(l)
A) 1.48 × 1024 molecules H2S
B) 9.89 × 1023 molecules H2S
C) 5.06 × 1025 molecules H2S
D) 3.17 × 1025 molecules H2S
E) 2.44 × 1023 molecules H2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
18
How many grams of Li3N can be formed from 1.75 moles of Li? Assume an excess of nitrogen.
6 Li(s)+ N2(g)→ 2 Li3N(s)
A) 18.3 g Li3N
B) 20.3 g Li3N
C) 58.3 g Li3N
D) 61.0 g Li3N
E) 15.1 g Li3N
6 Li(s)+ N2(g)→ 2 Li3N(s)
A) 18.3 g Li3N
B) 20.3 g Li3N
C) 58.3 g Li3N
D) 61.0 g Li3N
E) 15.1 g Li3N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
19
How many moles of nitrogen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol.
4 KNO3(s)→ 2 K2O(s)+ 2 N2(g)+ 5 O2(g)
A) 0.290 mol N2
B) 0.580 mol N2
C) 18.5 mol N2
D) 0.724 mol N2
E) 1.73 mol N2
4 KNO3(s)→ 2 K2O(s)+ 2 N2(g)+ 5 O2(g)
A) 0.290 mol N2
B) 0.580 mol N2
C) 18.5 mol N2
D) 0.724 mol N2
E) 1.73 mol N2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
20
A chemical change
A) occurs when methane gas is burned.
B) occurs when paper is shredded.
C) occurs when water is vaporized.
D) occurs when salt is dissolved in water.
E) occurs when powdered lemonade is stirred into water.
A) occurs when methane gas is burned.
B) occurs when paper is shredded.
C) occurs when water is vaporized.
D) occurs when salt is dissolved in water.
E) occurs when powdered lemonade is stirred into water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
21
Balance the following equation. B2H6(g)+ O2(g)→ B2O3(s)+ H2O(g)
What is the stoichiometric coefficient for oxygen?
A) 1
B) 2
C) 3
D) 4
E) 6
What is the stoichiometric coefficient for oxygen?
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
22
Why are the halogens among the most active nonmetals?
A) Their valence electrons are more effectively shielded.
B) All halogens have low electron affinities.
C) They all have smaller diameters than other nonmetals.
D) Their d orbitals are completely filled.
E) They only need one electron needed to attain a nobel gas configuration
A) Their valence electrons are more effectively shielded.
B) All halogens have low electron affinities.
C) They all have smaller diameters than other nonmetals.
D) Their d orbitals are completely filled.
E) They only need one electron needed to attain a nobel gas configuration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is one of the products of a combustion reaction?
A) graphite
B) hydrogen
C) oxygen
D) heat
E) ethanol
A) graphite
B) hydrogen
C) oxygen
D) heat
E) ethanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many grams of the excess reagent are left over if 37.8g of Cl2 react with 39.4 g of NaF?
Cl2(g)+ 2 NaF(aq)→ 2 NaCl(aq)+ F2(g)
A) 8.40 g
B) 29.4 g
C) 1.43 g
D) 12.6 g
E) 4.47 g
Cl2(g)+ 2 NaF(aq)→ 2 NaCl(aq)+ F2(g)
A) 8.40 g
B) 29.4 g
C) 1.43 g
D) 12.6 g
E) 4.47 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
25
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 8.000 moles of C8H18 with 4.000 moles of O2. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
A) 20.00%
B) 25.00%
C) 50.00%
D) 12.50%
A) 20.00%
B) 25.00%
C) 50.00%
D) 12.50%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
26
Determine the percent yield of a reaction that produces 28.65 g of Fe when 50.00 g of Fe2O3 react with excess Al according to the following reaction. Fe2O3(s)+ 2 Al(s)→ Al2O3(s)+ 2 Fe(s)
A) 61.03 %
B) 28.65 %
C) 57.30 %
D) 20.02 %
E) 81.93 %
A) 61.03 %
B) 28.65 %
C) 57.30 %
D) 20.02 %
E) 81.93 %

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
27
How many moles of SnCl2 will be produced if 85.3 g of FeCl3 is reacted with an excess of Sn for the following reaction: 2 FeCl3(s)+ 3 Sn(s)→ 3 SnCl2(s)+ 2 Fe(s)
A) 0.526 mol
B) 0.789 mol
C) 1.20 mol
D) 1.58 mol
E) 2.43 mol
A) 0.526 mol
B) 0.789 mol
C) 1.20 mol
D) 1.58 mol
E) 2.43 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the percent yield of H2 if 58.94g of methane yields 14.61g of H2 for the following reaction: CH4(g)+ 2 H2O(g)→ 4 H2(g)+ CO2(g)
A) 2.017%
B) 72.88%
C) 49.21%
D) 64.29%
E) 24.47%
A) 2.017%
B) 72.88%
C) 49.21%
D) 64.29%
E) 24.47%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the percent yield of C2H2 if 62.80g of water yields 15.38g of C2H2 using the following equation. CaC2(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ C2H2(g)
A) 13.84%
B) 33.90%
C) 91.47%
D) 48.10%
E) 68.52%
A) 13.84%
B) 33.90%
C) 91.47%
D) 48.10%
E) 68.52%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which should give the most vigorous reaction when dropped in water?
A) C
B) K
C) Zn
D) Ca
E) Si
A) C
B) K
C) Zn
D) Ca
E) Si

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
31
Determine the theoretical yield of ethanol when 100.0g g of glucose is used according to the following fermentation reaction. C6H12O6(aq)→ 2 C2H5OH(l)+ 2 CO2(g)
A) 51.09g
B) 35.21g
C) 87.32g
D) 68.34g
E) 45.37g
A) 51.09g
B) 35.21g
C) 87.32g
D) 68.34g
E) 45.37g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
32
All of the following are examples of chemical properties EXCEPT
A) the low pH of stomach acid.
B) chlorophyll being responsible for the green color of leaves.
C) heat being given off by the burning of natural gas in a kitchen stove.
D) the rusting of an iron nail left out in the rain.
E) the ability of a drain opener to unclog a drain using sodium hydroxide.
A) the low pH of stomach acid.
B) chlorophyll being responsible for the green color of leaves.
C) heat being given off by the burning of natural gas in a kitchen stove.
D) the rusting of an iron nail left out in the rain.
E) the ability of a drain opener to unclog a drain using sodium hydroxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
33
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 4.000 moles of C8H18 with 8.000 moles of O2. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
A) 20.00%
B) 25.00%
C) 50.00%
D) 12.50%
A) 20.00%
B) 25.00%
C) 50.00%
D) 12.50%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
34
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 4.000 moles of C8H18 with 4.000 moles of O2. 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
A) 20.00%
B) 25.00%
C) 50.00%
D) 12.50%
A) 20.00%
B) 25.00%
C) 50.00%
D) 12.50%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which should give the least vigorous reaction when dropped in water?
A) Li
B) Na
C) K
D) Rb
E) Cs
A) Li
B) Na
C) K
D) Rb
E) Cs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which one of the following halogen reaction contains a product with covalent bonds?
A) Mg(s) + F2(g) → MgF2(s)
B) C(s) + O2(g) → CO2(g)
C) 2Cu(s) + I2(g) → 2CuI(s)
D) Br2(l) + Cl2(g) → 2BrCl(g)
E) V(s) + 2Cl2(g) → VCl4(l)
A) Mg(s) + F2(g) → MgF2(s)
B) C(s) + O2(g) → CO2(g)
C) 2Cu(s) + I2(g) → 2CuI(s)
D) Br2(l) + Cl2(g) → 2BrCl(g)
E) V(s) + 2Cl2(g) → VCl4(l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
37
Aluminum metal reacts with aqueous iron( III)oxide to form aqueous aluminum oxide and iron metal.What is the stoichiometric coefficient for aluminum when the chemical equation is balanced using the lowest,whole-number stoichiometric coefficients?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
38
According to the following reaction,what amount of Al2S3 remains when 20.00 g of Al2S3 and 2.00 g of H2O are reacted? A few of the molar masses are as follows: Al2S3 = 150.17 g/mol,H2O = 18.02 g/mol.
Al2S3(s)+ 6 H2O(l)→ 2 Al(OH)3(s)+ 3 H2S(g)
A) 28.33 g
B) 14.00 g
C) 8.33 g
D) 19.78 g
E) 17.22 g
Al2S3(s)+ 6 H2O(l)→ 2 Al(OH)3(s)+ 3 H2S(g)
A) 28.33 g
B) 14.00 g
C) 8.33 g
D) 19.78 g
E) 17.22 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
39
Choose the reaction that represents the combustion of C6H12O2.
A) C6H12O2(l) + 8 O2(g) → 6 CO2(g) + 6 H2O(g)
B) Mg(s) + C6H12O2(l) → MgC6H12O2(aq)
C) 6 C(s) + 6 H2(g) + O2(g) → C6H12O2(l)
D) C6H12O2(l) → 6 C(s) + 6 H2(g) + O2(g)
E) None of the above represent the combustion of C6H12O2.
A) C6H12O2(l) + 8 O2(g) → 6 CO2(g) + 6 H2O(g)
B) Mg(s) + C6H12O2(l) → MgC6H12O2(aq)
C) 6 C(s) + 6 H2(g) + O2(g) → C6H12O2(l)
D) C6H12O2(l) → 6 C(s) + 6 H2(g) + O2(g)
E) None of the above represent the combustion of C6H12O2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
40
How many moles of PCl5 will be produced if .331 moles of P4 is reacted with an excess of Cl2 using the following equation. P4(s)+ 10 Cl2(g)→ 4 PCl5(g)
A) .331 mol
B) .662 mol
C) .993 mol
D) 1.324 mol
E) 1.655 mol
A) .331 mol
B) .662 mol
C) .993 mol
D) 1.324 mol
E) 1.655 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
41
How many moles of BCl3 are needed to produce 5.00 g of HCl(aq)in the following reaction?
BCl3(g)+ 3 H2O(l)→ 3 HCl(aq)+ B(OH)3(aq)
A) 0. 0457 mol
B) 0. 137 mol
C) 0.411 mol
D) 21.9 mol
BCl3(g)+ 3 H2O(l)→ 3 HCl(aq)+ B(OH)3(aq)
A) 0. 0457 mol
B) 0. 137 mol
C) 0.411 mol
D) 21.9 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
42
According to the following balanced reaction,how many moles of KO are required to exactly react with 4.33 moles of H2O?
4 KO(s)+ 2 H2O(l)→ 4 KOH(s)+ O2(g)
A) 4.33 moles H2O
B) 8.66 moles H2O
C) 17.3 moles H2O
D) 2.17 moles H2O
E) 1.08 moles H2O
4 KO(s)+ 2 H2O(l)→ 4 KOH(s)+ O2(g)
A) 4.33 moles H2O
B) 8.66 moles H2O
C) 17.3 moles H2O
D) 2.17 moles H2O
E) 1.08 moles H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
43
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 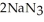 (s)→ 2Na(s)+
(s)→ 2Na(s)+ 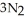 (g) How many moles of
(g) How many moles of 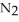 are produced by the decomposition of 1.25 mol of sodium azide?
are produced by the decomposition of 1.25 mol of sodium azide?
A) 0.833
B) 3.75
C) 1.88
D) 0.417
E) 0.625
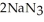 (s)→ 2Na(s)+
(s)→ 2Na(s)+ 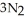 (g) How many moles of
(g) How many moles of 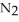 are produced by the decomposition of 1.25 mol of sodium azide?
are produced by the decomposition of 1.25 mol of sodium azide?A) 0.833
B) 3.75
C) 1.88
D) 0.417
E) 0.625

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
44
Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.How many grams of oxygen are formed when 20.0 g of dinitrogen monoxide decomposes?
A) 0. 138 g
B) 7.27 g
C) 14.5 g
D) 29.1 g
A) 0. 138 g
B) 7.27 g
C) 14.5 g
D) 29.1 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
45
Carbonic acid can form water and carbon dioxide upon heating.How much carbon dioxide is formed from 12.4 g of carbonic acid?
H2CO3 → H2O + CO2
A) 8.80 g
B) 17.5 g
C) 12.4 g
D) 3.60 g
E) 42.7 g
H2CO3 → H2O + CO2
A) 8.80 g
B) 17.5 g
C) 12.4 g
D) 3.60 g
E) 42.7 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many molecules of HCl are formed when 90.0 g of water reacts according to the following balanced reaction? Assume excess ICl3.
2 ICl3 + 3 H2O → ICl + HIO3 + 5 HCl
A) 5.00 × 1024 molecules HCl
B) 3.00 × 1024 molecules HCl
C) 9.00 × 1024 molecules HCl
D) 6.00 × 1024 molecules HCl
E) 5.00 × 1025 molecules HCl
2 ICl3 + 3 H2O → ICl + HIO3 + 5 HCl
A) 5.00 × 1024 molecules HCl
B) 3.00 × 1024 molecules HCl
C) 9.00 × 1024 molecules HCl
D) 6.00 × 1024 molecules HCl
E) 5.00 × 1025 molecules HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
47
Balance the chemical equation given below,and determine the number of grams of MgO are needed to produce 15.0 g of Fe2O3. ________ MgO(s)+ ________ Fe(s)→ ________ Fe2O3(s)+ ________ Mg(s)
A) 0.0877 g
B) 1.26 g
C) 3.78 g
D) 11.4 g
A) 0.0877 g
B) 1.26 g
C) 3.78 g
D) 11.4 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
48
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2Mg(s)+ 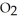 (g)→ 2MgO(s)
(g)→ 2MgO(s)
How many moles of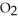 are consumed when 1.90 mol of magnesium burns?
are consumed when 1.90 mol of magnesium burns?
A) 0.0782
B) 1.05
C) 1.90
D) 3.80
E) 0.950
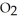 (g)→ 2MgO(s)
(g)→ 2MgO(s)How many moles of
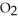 are consumed when 1.90 mol of magnesium burns?
are consumed when 1.90 mol of magnesium burns?A) 0.0782
B) 1.05
C) 1.90
D) 3.80
E) 0.950

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many grams of oxygen are formed when 5.30 moles of KOH are formed?
4 KO(s)+ 2 H2O(l)→ 4 KOH(s)+ O2(g)
A) 5.30 g O2
B) 21.2 g O2
C) 42.4 g O2
D) 170 g O2
E) 84.8 g O2
4 KO(s)+ 2 H2O(l)→ 4 KOH(s)+ O2(g)
A) 5.30 g O2
B) 21.2 g O2
C) 42.4 g O2
D) 170 g O2
E) 84.8 g O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
50
Lithium and nitrogen react to produce lithium nitride: 6Li(s)+ 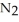 (g)→
(g)→ 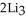 N(s)
N(s)
How many moles of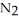 are needed to react with 0.450 mol of lithium?
are needed to react with 0.450 mol of lithium?
A) 2.70
B) 0.450
C) 0.150
D) 1.35
E) 0.0750
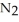 (g)→
(g)→ 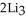 N(s)
N(s)How many moles of
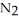 are needed to react with 0.450 mol of lithium?
are needed to react with 0.450 mol of lithium?A) 2.70
B) 0.450
C) 0.150
D) 1.35
E) 0.0750

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
51
According to the following balanced reaction,how many moles of NO are formed from 8.44 moles of NO2 if there is plenty of water present?
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A) 25.32 moles NO
B) 12.66 moles NO
C) 8.44 moles NO
D) 5.63 moles NO
E) 2.82 moles NO
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A) 25.32 moles NO
B) 12.66 moles NO
C) 8.44 moles NO
D) 5.63 moles NO
E) 2.82 moles NO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
52
Lithium and nitrogen react to produce lithium nitride: 6Li(s)+ 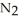 (g)→
(g)→ 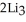 N(s)
N(s)
How many moles of lithium nitride are produced when 0.550 mol of lithium react in this fashion?
A) 0.183
B) 1.10
C) 0.0917
D) 1.65
E) 0.275
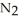 (g)→
(g)→ 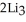 N(s)
N(s)How many moles of lithium nitride are produced when 0.550 mol of lithium react in this fashion?
A) 0.183
B) 1.10
C) 0.0917
D) 1.65
E) 0.275

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
53
If the density of ethanol,C2H5OH,is 0.789 g/mL.How many milliliters of ethanol are needed to produce 20.0 g of CO2 according to the following chemical equation?
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
A) 8.26 mL
B) 13.3 mL
C) 26.5 mL
D) 53.1 mL
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
A) 8.26 mL
B) 13.3 mL
C) 26.5 mL
D) 53.1 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
54
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 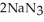 (s)→ 2Na(s)+
(s)→ 2Na(s)+ 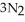 (g) How many grams of sodium azide are required to produce 25.0 g of nitrogen?
(g) How many grams of sodium azide are required to produce 25.0 g of nitrogen?
A) 1.34
B) 0.595
C) 58.0
D) 38.7
E) 87.0
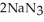 (s)→ 2Na(s)+
(s)→ 2Na(s)+ 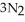 (g) How many grams of sodium azide are required to produce 25.0 g of nitrogen?
(g) How many grams of sodium azide are required to produce 25.0 g of nitrogen?A) 1.34
B) 0.595
C) 58.0
D) 38.7
E) 87.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
55
Consider the following reaction.How many moles of oxygen are required to produce 4.00 moles of water? Assume that there is excess C3H7SH present.
C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
A) 2.67 moles O2
B) 6.00 moles O2
C) 4.00 moles O2
D) 16.0 moles O2
E) 1.00 moles O2
C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
A) 2.67 moles O2
B) 6.00 moles O2
C) 4.00 moles O2
D) 16.0 moles O2
E) 1.00 moles O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
56
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s)+ 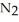 (g)→
(g)→ 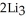 N(s)
N(s)
How many moles of lithium are needed to produce 0.45 mol of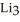 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
A) 0.23
B) 1.4
C) 0.15
D) 0.30
E) 2.7
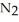 (g)→
(g)→ 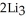 N(s)
N(s)How many moles of lithium are needed to produce 0.45 mol of
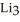 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?A) 0.23
B) 1.4
C) 0.15
D) 0.30
E) 2.7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
57
Strontium phosphate reacts with sulfuric acid to form strontium sulfate and phosphoric acid.What is the coefficient for sulfuric acid when the equation is balanced using the lowest,whole-numbered coefficients?
A) 1
B) 2
C) 3
D) none of these
A) 1
B) 2
C) 3
D) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
58
Balance the chemical equation given below,and determine the number of moles of iodine that reacts with 20.0 g of aluminum. ________ Al(s)+ ________ I2(s)→ ________ Al2I6(s)
A) 0.494 mol
B) 1.11 mol
C) 1.48 mol
D) 2.22 mol
A) 0.494 mol
B) 1.11 mol
C) 1.48 mol
D) 2.22 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
59
How many moles of CuO can be produced from 2.25 mol of Cu2O in the following reaction?
2 Cu2O(s)+ O2(g)→ 4 CuO(s)
A) 1.12 mol
B) 2.25 mol
C) 4.50 mol
D) 9.00 mol
2 Cu2O(s)+ O2(g)→ 4 CuO(s)
A) 1.12 mol
B) 2.25 mol
C) 4.50 mol
D) 9.00 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
60
How many grams of calcium chloride are needed to produce 5.00 g of potassium chloride?
CaCl2(aq)+ K2CO3(aq)→ 2 KCl(aq)+ CaCO3(aq)
A) 0.269 g
B) 3.72 g
C) 7.44 g
D) 14.9 g
CaCl2(aq)+ K2CO3(aq)→ 2 KCl(aq)+ CaCO3(aq)
A) 0.269 g
B) 3.72 g
C) 7.44 g
D) 14.9 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
61
Determine the theoretical yield of H2S (in moles)if 16 mol Al2S3 and 16 mol H2O are reacted according to the following balanced reaction.A possibly useful molar mass is Al2S3 = 150.17 g/mol. Al2S3(s)+ 6 H2O(l)→ 2 Al(OH)3(s)+ 3 H2S(g)
A) 48 mol H2S
B) 16 mol H2S
C) 32 mol H2S
D) 24 mol H2S
E) 8.0 mol H2S
A) 48 mol H2S
B) 16 mol H2S
C) 32 mol H2S
D) 24 mol H2S
E) 8.0 mol H2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
62
Sodium metal and water react to form hydrogen and sodium hydroxide.If 17.94 g of sodium react with water to form 0.78 g of hydrogen and 31.20 g of sodium hydroxide,what mass of water was involved in the reaction?
A) 14.04 g
B) 17.94 g
C) 30.42 g
D) 31.98 g
A) 14.04 g
B) 17.94 g
C) 30.42 g
D) 31.98 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
63
When 14.0 g of calcium metal is reacted with water,5.00 g of calcium hydroxide is produced.Using the following balanced equation,calculate the percent yield for the reaction?
Ca(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ H2(g)
A) 9.67%
B) 19.3%
C) 35.7%
D) 66.0%
Ca(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ H2(g)
A) 9.67%
B) 19.3%
C) 35.7%
D) 66.0%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
64
Describe the greenhouse effect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
65
When silver nitrate reacts with barium chloride,silver chloride and barium nitrate are formed.How many grams of silver chloride are formed when 10.2 g of silver nitrate reacts with 15.0 g of barium chloride?
A) 8.61 g
B) 9.40 g
C) 12.1 g
D) 18.8 g
A) 8.61 g
B) 9.40 g
C) 12.1 g
D) 18.8 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
66
Balance the following equation.
________ C10H12 + ________ O2 → ________ H2O + ________ CO2
________ C10H12 + ________ O2 → ________ H2O + ________ CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which substance is the limiting reactant when 8.0 g of sulfur reacts with 12 g of oxygen and 16 g of sodium hydroxide according to the following chemical equation: 2 S(s)+ 3 O2(g)+ 4 NaOH(aq)→ 2 Na2SO4(aq)+ 2 H2O(l)
A) S(s)
B) O2(g)
C) NaOH(aq)
D) None of these substances is the limiting reactant.
A) S(s)
B) O2(g)
C) NaOH(aq)
D) None of these substances is the limiting reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
68
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s)+ 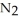 (g)→
(g)→ 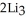 N(s)
N(s)
In a particular experiment,1.00-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
A) 1.01
B) 0.84
C) 5.0
D) 1.67
E) 2.50
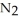 (g)→
(g)→ 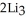 N(s)
N(s)In a particular experiment,1.00-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
A) 1.01
B) 0.84
C) 5.0
D) 1.67
E) 2.50

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
69
If the percent yield for the following reaction is 75.0%,and 25.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq)are produced?
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A) 17.1 g
B) 22.8 g
C) 30.4 g
D) 38.5 g
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
A) 17.1 g
B) 22.8 g
C) 30.4 g
D) 38.5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
70
8.0 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is FALSE?
N2(g)+ 3 H2(g)→ 2 NH3(g)
A) 3.3 g of hydrogen are left over.
B) Hydrogen is the excess reactant.
C) Nitrogen is the limiting reactant.
D) The theoretical yield of ammonia is 15 g.
N2(g)+ 3 H2(g)→ 2 NH3(g)
A) 3.3 g of hydrogen are left over.
B) Hydrogen is the excess reactant.
C) Nitrogen is the limiting reactant.
D) The theoretical yield of ammonia is 15 g.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
71
If 588 grams of FeS2 is allowed to react with 352 grams of O2 according to the following unbalanced equation,how many grams of Fe2O3 are produced?
FeS2 + O2 → Fe2O3 + SO2
FeS2 + O2 → Fe2O3 + SO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
72
9.0 g of iron is reacted with 9.0 g of water according to the chemical equation shown below.Which one of the following statements is FALSE?
3 Fe(s)+ 4 H2O(l)→ Fe3O4(s)+ 4 H2(g)
A) 12.4 g of Fe3O4 are produced.
B) 5.13 g of H2O are left over.
C) Mass is conserved in this reaction.
D) Water is the limiting reactant.
3 Fe(s)+ 4 H2O(l)→ Fe3O4(s)+ 4 H2(g)
A) 12.4 g of Fe3O4 are produced.
B) 5.13 g of H2O are left over.
C) Mass is conserved in this reaction.
D) Water is the limiting reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
73
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s)+ 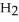 O(l)→
O(l)→ 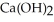 (s)
(s)
A 5.00-g sample of CaO is reacted with 4.83 g of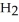 O.How many grams of water remain after the reaction is complete?
O.How many grams of water remain after the reaction is complete?
A) 0.00
B) 0.00991
C) 3.22
D) 1.04
E) 0.179
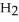 O(l)→
O(l)→ 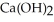 (s)
(s)A 5.00-g sample of CaO is reacted with 4.83 g of
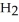 O.How many grams of water remain after the reaction is complete?
O.How many grams of water remain after the reaction is complete?A) 0.00
B) 0.00991
C) 3.22
D) 1.04
E) 0.179

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
74
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s)+ 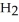 O(l)→
O(l)→ 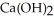 (s)
(s)
In a particular experiment,a 5.50-g sample of CaO is reacted with excess water and 6.77 g of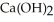 is recovered.What is the percent yield in this experiment?
is recovered.What is the percent yield in this experiment?
A) 123
B) 1.23
C) 7.91
D) 93.1
E) 81.3
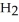 O(l)→
O(l)→ 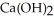 (s)
(s)In a particular experiment,a 5.50-g sample of CaO is reacted with excess water and 6.77 g of
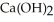 is recovered.What is the percent yield in this experiment?
is recovered.What is the percent yield in this experiment?A) 123
B) 1.23
C) 7.91
D) 93.1
E) 81.3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
75
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2Mg(s)+ 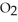 (g)→ 2MgO(s)
(g)→ 2MgO(s)
When 3.00 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
A) 3.00
B) 4.98
C) 0.123
D) 2.49
E) 10.0
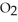 (g)→ 2MgO(s)
(g)→ 2MgO(s)When 3.00 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
A) 3.00
B) 4.98
C) 0.123
D) 2.49
E) 10.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
76
Balance the following equation.
________ C9H20 + ________ O2 → ________ H2O + ________ CO2
________ C9H20 + ________ O2 → ________ H2O + ________ CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
77
Define a limiting reagent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
78
When 8.00 × 1022 molecules of ammonia react with 7.00 × 1022 molecules of oxygen according to the chemical equation shown below,how many grams of nitrogen gas are produced?
4 NH3(g)+ 3 O2(g)→ 2 N2(g)+ 6 H2O(g)
A) 1.86 g
B) 2.17 g
C) 4.88 g
D) 7.44 g
4 NH3(g)+ 3 O2(g)→ 2 N2(g)+ 6 H2O(g)
A) 1.86 g
B) 2.17 g
C) 4.88 g
D) 7.44 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck
79
If the percent yield for the following reaction is 65.0%,how many grams of KClO3 are needed to produce 42.0 g of O2?
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A) 69.7 g
B) 107 g
C) 165 g
D) 371 g
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A) 69.7 g
B) 107 g
C) 165 g
D) 371 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 79 في هذه المجموعة.
فتح الحزمة
k this deck








