Deck 22: Organic Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/105
العب
ملء الشاشة (f)
Deck 22: Organic Chemistry
1
Nonsuperimposable mirror images are called
A) structural isomers.
B) achiral.
C) diastereomers.
D) enantiomers.
E) racemic mixture.
A) structural isomers.
B) achiral.
C) diastereomers.
D) enantiomers.
E) racemic mixture.
enantiomers.
2
Name the following compound. 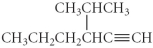
A) 4-propyl-5-hexyne
B) 3-isopropyl-1-hexyne
C) 1-nonyne
D) 4-methyl-3-propyl-1-pentyne
E) 2-methyl-4-pentyne
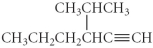
A) 4-propyl-5-hexyne
B) 3-isopropyl-1-hexyne
C) 1-nonyne
D) 4-methyl-3-propyl-1-pentyne
E) 2-methyl-4-pentyne
3-isopropyl-1-hexyne
3
Name the following compound. 
A) 4-methyl-4-propylpentane
B) 4,4-dimethylheptane
C) nonane
D) 4-propyl-4-methylpentane
E) 2-methyl-2-propylpentane

A) 4-methyl-4-propylpentane
B) 4,4-dimethylheptane
C) nonane
D) 4-propyl-4-methylpentane
E) 2-methyl-2-propylpentane
4,4-dimethylheptane
4
Name the following compound. 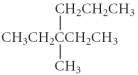
A) 3-ethyl-3-methylhexane
B) 3-methyl-3-propylpentane
C) 3-ethyl-3-propylbutane
D) nonane
E) 2-ethylheptane
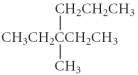
A) 3-ethyl-3-methylhexane
B) 3-methyl-3-propylpentane
C) 3-ethyl-3-propylbutane
D) nonane
E) 2-ethylheptane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
5
An equimolar mixture of two optical isomers is called a
A) structural isomer.
B) achiral.
C) diastereomer.
D) enantiomer.
E) racemic mixture.
A) structural isomer.
B) achiral.
C) diastereomer.
D) enantiomer.
E) racemic mixture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
6
Molecules with the same formula but different structures are called
A) structural isomers.
B) achiral.
C) diastereomers.
D) enantiomers.
E) racemic mixture.
A) structural isomers.
B) achiral.
C) diastereomers.
D) enantiomers.
E) racemic mixture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
7
Identify the formula for an alkane.
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following names is correct?
A) 3-methyl-5-heptyne
B) 2-ethyl-4-hexyne
C) 5-ethyl-2-hexyne
D) 4-butyl-1-pentyne
E) 5-methyl-2-heptyne
A) 3-methyl-5-heptyne
B) 2-ethyl-4-hexyne
C) 5-ethyl-2-hexyne
D) 4-butyl-1-pentyne
E) 5-methyl-2-heptyne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
9
Identify the formula for an alkene.
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
10
How many of the carbons in the following compound are chiral center(s)? 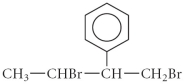
A) 0
B) 1
C) 2
D) 3
E) 4 or more

A) 0
B) 1
C) 2
D) 3
E) 4 or more

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
11
Give the number of covalent bonds that a carbon atom can form.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following compounds exhibit geometric isomerism?
A) CH2=CH-CH3
B) CCl2=CBr2
C) CH3-CH=CH-CH3
D) CCl2=CHBr
E) All of the above exhibit geometric isomerism.
A) CH2=CH-CH3
B) CCl2=CBr2
C) CH3-CH=CH-CH3
D) CCl2=CHBr
E) All of the above exhibit geometric isomerism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
13
Identify the formula for an alkyne.
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
14
Name the following compound. 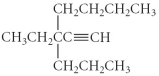
A) 3-butyl-3-propyl-1-pentyne
B) 3-butyl-3-propyl-4-pentyne
C) 3-ethyl-3-propyl-1-heptyne
D) 5-ethyl-5-propyl-6-heptyne
E) 3-ethyl-3-butyl-1-hexyne
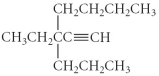
A) 3-butyl-3-propyl-1-pentyne
B) 3-butyl-3-propyl-4-pentyne
C) 3-ethyl-3-propyl-1-heptyne
D) 5-ethyl-5-propyl-6-heptyne
E) 3-ethyl-3-butyl-1-hexyne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
15
How many of the carbons in the following compound are chiral center(s)? 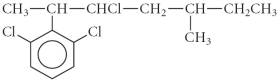
A) 0
B) 1
C) 2
D) 3
E) 4 or more
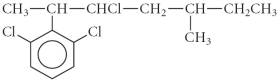
A) 0
B) 1
C) 2
D) 3
E) 4 or more

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following names is correct?
A) 3-butyl-4-pentene
B) 3-ethyl-1-heptene
C) 3-etheneheptane
D) 5-ethyl-6-heptene
E) All of the above are correct.
A) 3-butyl-4-pentene
B) 3-ethyl-1-heptene
C) 3-etheneheptane
D) 5-ethyl-6-heptene
E) All of the above are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
17
Name the following compound. 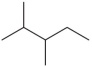
A) 2-methyl-3-methylhexane
B) heptane
C) 2,3-dimethylpentane
D) 3,4-dimethylpentane
E) 2,4-dimethylpropane

A) 2-methyl-3-methylhexane
B) heptane
C) 2,3-dimethylpentane
D) 3,4-dimethylpentane
E) 2,4-dimethylpropane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following names is correct?
A) 2-methyl-4-pentyne
B) 3-ethyl-1-butyne
C) 3-butyl-3-propyl-1-pentyne
D) 3-methyl-1-butyne
E) 3-ethyl-3-butyl-1-hexyne
A) 2-methyl-4-pentyne
B) 3-ethyl-1-butyne
C) 3-butyl-3-propyl-1-pentyne
D) 3-methyl-1-butyne
E) 3-ethyl-3-butyl-1-hexyne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
19
Name the following compound. 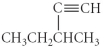
A) 3-methyl-1-pentyne
B) 3-methyl-4-pentyne
C) 2-ethynebutane
D) 1-hexyne
E) 3-ethyl-1-butyne
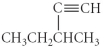
A) 3-methyl-1-pentyne
B) 3-methyl-4-pentyne
C) 2-ethynebutane
D) 1-hexyne
E) 3-ethyl-1-butyne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
20
Any molecule that exhibits optical isomerism is said to be
A) a stereoisomer.
B) a hydrocarbon.
C) chiral.
D) an alkyne.
E) biologically active.
A) a stereoisomer.
B) a hydrocarbon.
C) chiral.
D) an alkyne.
E) biologically active.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
21
Write the balanced chemical equation for the addition of 2 Br2 to CH3C≡CH.
A) CH3C≡CH + 2 Br2 → CH3CBr2CHBr2
B) CH3C≡CH + 2 Br2 → CH3CBr=CHBr
C) CH3C≡CH + 2 Br2 → CH3CH2CHBr2
D) CH3C≡CH + 2 Br2 → CH3CBr2CH3
E) CH3C≡CH + 2 Br2 → CH3CHBrCH2Br
A) CH3C≡CH + 2 Br2 → CH3CBr2CHBr2
B) CH3C≡CH + 2 Br2 → CH3CBr=CHBr
C) CH3C≡CH + 2 Br2 → CH3CH2CHBr2
D) CH3C≡CH + 2 Br2 → CH3CBr2CH3
E) CH3C≡CH + 2 Br2 → CH3CHBrCH2Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following compounds is ethanol?
A) C2H6
B) C2H5OH
C) CH3CO2H
D) CH3CO2CH3
E) CH3-O-CH3
A) C2H6
B) C2H5OH
C) CH3CO2H
D) CH3CO2CH3
E) CH3-O-CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
23
Write the balanced chemical equation for the catalytic hydrogenation (addition of H2)to CH3CH=CHCH3.
A) CH3CH=CHCH3 + 2 H2 → 2 CH3CH3
B) CH3CH=CHCH3 + 3 H2 → CH3CH3 + 2 CH4
C) CH3CH=CHCH3 + 2 H2 → CH3CH2CH3 + CH4
D) CH3CH=CHCH3 + H2 → CH3CH2CH2CH3
E) CH3CH=CHCH3 + 4 H2 → 4 CH4
A) CH3CH=CHCH3 + 2 H2 → 2 CH3CH3
B) CH3CH=CHCH3 + 3 H2 → CH3CH3 + 2 CH4
C) CH3CH=CHCH3 + 2 H2 → CH3CH2CH3 + CH4
D) CH3CH=CHCH3 + H2 → CH3CH2CH2CH3
E) CH3CH=CHCH3 + 4 H2 → 4 CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
24
Name the following compound. 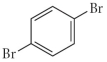
A) 2,5-dibromobenzene
B) 2,5-dibromocyclohexene
C) 1,4-dibromobenzene
D) 1,4-bromocyclohexene
E) 3,6-dibromobenzene

A) 2,5-dibromobenzene
B) 2,5-dibromocyclohexene
C) 1,4-dibromobenzene
D) 1,4-bromocyclohexene
E) 3,6-dibromobenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
25
Write a balanced chemical reaction to represent the combustion of 2,2-dimethylpropane.
A) C5H12 + 8 O2 → 5 CO2 + 6 H2O
B) C3H8 + 5 O2 → 3 CO2 + 4 H2O
C) C5H12 + H2 → CH4 + 2 C2H6
D) C3H8 + H2 → CH4 + C2H6
E) 2 C3H8 + O2 → 3 CH4 + 2 H2O
A) C5H12 + 8 O2 → 5 CO2 + 6 H2O
B) C3H8 + 5 O2 → 3 CO2 + 4 H2O
C) C5H12 + H2 → CH4 + 2 C2H6
D) C3H8 + H2 → CH4 + C2H6
E) 2 C3H8 + O2 → 3 CH4 + 2 H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following compounds is an ether?
A)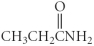
B)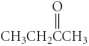
C)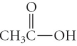
D)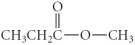
E)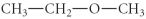
A)
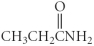
B)
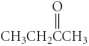
C)
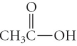
D)
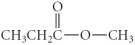
E)
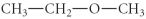

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following compounds is an alcohol?
A) CH3CH2CH2CO2H
B) CH3-O-CH3
C) CH3CO2CH3
D) CH3CH2CH=O
E) CH2OH-CH2OH
A) CH3CH2CH2CO2H
B) CH3-O-CH3
C) CH3CO2CH3
D) CH3CH2CH=O
E) CH2OH-CH2OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
28
Write the balanced chemical equation that represents the addition of Cl2 to CH3CH=CH2.
A) CH3CH=CH2 + Cl2 → CH3CHClCH3 + HCl
B) CH3CH=CH2 + Cl2 → CH3CCl=CHCl + H2
C) CH3CH=CH2 + Cl2 → CH3CHClCH2Cl
D) CH3CH=CH2 + 2 Cl2 → CH3CHCl2 + CH2Cl2
E) CH3CH=CH2 + 2 Cl2 → CH3CCl2CHCl2 + H2
A) CH3CH=CH2 + Cl2 → CH3CHClCH3 + HCl
B) CH3CH=CH2 + Cl2 → CH3CCl=CHCl + H2
C) CH3CH=CH2 + Cl2 → CH3CHClCH2Cl
D) CH3CH=CH2 + 2 Cl2 → CH3CHCl2 + CH2Cl2
E) CH3CH=CH2 + 2 Cl2 → CH3CCl2CHCl2 + H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following compounds is an aldehyde?
A) CH3CH2CH=O
B) CH3CH2CH2CO2H
C) CH3CO2CH3
D) CH3-O-CH3
E) CH2OH-CH2OH
A) CH3CH2CH=O
B) CH3CH2CH2CO2H
C) CH3CO2CH3
D) CH3-O-CH3
E) CH2OH-CH2OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
30
Complete and balance the following addition reaction. CH3C  CCH3 + 2 Cl2 → ?
CCH3 + 2 Cl2 → ?
A) CH3C CCH3 + 2 Cl2 → CH3CCl2CCl2CH3
CCH3 + 2 Cl2 → CH3CCl2CCl2CH3
B) CH3C CCH3 + 3 Cl2 → 2 CH3CCl3
CCH3 + 3 Cl2 → 2 CH3CCl3
C) CH3C CCH3 + 2 Cl2 → 4 CH3Cl
CCH3 + 2 Cl2 → 4 CH3Cl
D) CH3C CCH3 + 4 Cl2 → 4 CH2Cl2
CCH3 + 4 Cl2 → 4 CH2Cl2
E) CH3C CCH3 + Cl2 → CH3CHClCHClCH3
CCH3 + Cl2 → CH3CHClCHClCH3
 CCH3 + 2 Cl2 → ?
CCH3 + 2 Cl2 → ?A) CH3C
 CCH3 + 2 Cl2 → CH3CCl2CCl2CH3
CCH3 + 2 Cl2 → CH3CCl2CCl2CH3B) CH3C
 CCH3 + 3 Cl2 → 2 CH3CCl3
CCH3 + 3 Cl2 → 2 CH3CCl3C) CH3C
 CCH3 + 2 Cl2 → 4 CH3Cl
CCH3 + 2 Cl2 → 4 CH3ClD) CH3C
 CCH3 + 4 Cl2 → 4 CH2Cl2
CCH3 + 4 Cl2 → 4 CH2Cl2E) CH3C
 CCH3 + Cl2 → CH3CHClCHClCH3
CCH3 + Cl2 → CH3CHClCHClCH3
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following compounds is a ketone?
A) CH3CH2CH2CO2H
B)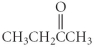
C) CH3CH2NH2
D)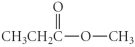
E)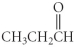
A) CH3CH2CH2CO2H
B)
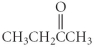
C) CH3CH2NH2
D)
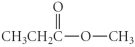
E)
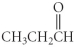

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
32
Write the balanced chemical equation for the catalytic hydrogenation (addition of H2)to CH3C≡CH.
A) CH3C≡CH + H2 → CH3CH=CH2
B) CH3C≡CH + 2 H2 → CH3CH=CH2
C) CH3C≡CH + H2 → CH3CH2CH3
D) CH3C≡CH + 2 H2 → CH3CH2CH3
E) CH3C≡CH + H2 → CH2=C=CH2
A) CH3C≡CH + H2 → CH3CH=CH2
B) CH3C≡CH + 2 H2 → CH3CH=CH2
C) CH3C≡CH + H2 → CH3CH2CH3
D) CH3C≡CH + 2 H2 → CH3CH2CH3
E) CH3C≡CH + H2 → CH2=C=CH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
33
Name the following compound. 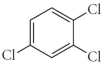
A) 2,3,5-trichlorobenzene
B) 1,3,4-trichlorobenzene
C) trichlorostyrene
D) 1,2,4-trichlorobenzene
E) 1,3,4-trichlorohexene

A) 2,3,5-trichlorobenzene
B) 1,3,4-trichlorobenzene
C) trichlorostyrene
D) 1,2,4-trichlorobenzene
E) 1,3,4-trichlorohexene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
34
Write the balanced chemical equation for the addition of HBr to CH2=CHCH2CH3.
A) CH2=CHCH2CH3 + HBr → CH3BrCH2CH2CH3
B) CH2=CHCH2CH3 + 2 HBr → 2 CH2BrCH3
C) CH2=CHCH2CH3 + 2 HBr → CH3Br + CH2BrCH2CH3
D) CH2=CHCH2CH3 + 4 HBr → 4 CH3Br
E) CH2=CHCH2CH3 + HBr → CH3CHBrCH2CH3
A) CH2=CHCH2CH3 + HBr → CH3BrCH2CH2CH3
B) CH2=CHCH2CH3 + 2 HBr → 2 CH2BrCH3
C) CH2=CHCH2CH3 + 2 HBr → CH3Br + CH2BrCH2CH3
D) CH2=CHCH2CH3 + 4 HBr → 4 CH3Br
E) CH2=CHCH2CH3 + HBr → CH3CHBrCH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following compounds exhibits geometric isomerism?
A) CH2=CH2
B) CH2=CCl2
C) CBr2=CHBr
D) CHCl=CHCl
E) (CH3)2C=CH-CH3
A) CH2=CH2
B) CH2=CCl2
C) CBr2=CHBr
D) CHCl=CHCl
E) (CH3)2C=CH-CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
36
Name the following compound. 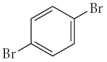
A) o-dibromobenzene
B) m-dibromobenzene
C) p-dibromobenzene
D) p-bromobenzene
E) none of the above

A) o-dibromobenzene
B) m-dibromobenzene
C) p-dibromobenzene
D) p-bromobenzene
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following compounds is an ester?
A)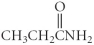
B)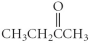
C)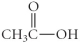
D)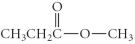
E)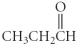
A)
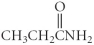
B)
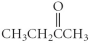
C)
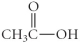
D)
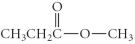
E)
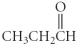

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following compounds is a carboxylic acid?
A)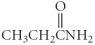
B)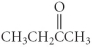
C)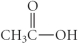
D)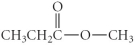
E)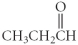
A)
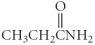
B)
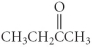
C)
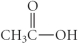
D)
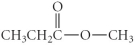
E)
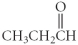

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
39
Write a balanced chemical equation to represent the reaction of 3-ethyl-2-methylhexane with Br2.
A) C7H14 + Br2 → C7H12Br + HBr
B) C9H20 + Br2 → C9H19Br + HBr
C) C6H12 + Br2 → C6H10Br2 + H2
D) C9H18 + Br2 → C9H16Br2 + H2
E) C6H12 + Br2 → C6H11Br + HBr
A) C7H14 + Br2 → C7H12Br + HBr
B) C9H20 + Br2 → C9H19Br + HBr
C) C6H12 + Br2 → C6H10Br2 + H2
D) C9H18 + Br2 → C9H16Br2 + H2
E) C6H12 + Br2 → C6H11Br + HBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
40
Complete and balance the following complete hydrogenation reaction. CH3CH2C  CCH3 + 2 H2 → ?
CCH3 + 2 H2 → ?
A) CH3CH2C CCH3 + 3 H2 → CH3CH2CH3 + CH3CH3
CCH3 + 3 H2 → CH3CH2CH3 + CH3CH3
B) CH3CH2C CCH3 + 2 H2 → CH3CH2CH2CH2CH3
CCH3 + 2 H2 → CH3CH2CH2CH2CH3
C) CH3CH2C CCH3 + 6 H2 → 5 CH4
CCH3 + 6 H2 → 5 CH4
D) 2 CH3CH2C CCH3 + 2 H2 → CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
CCH3 + 2 H2 → CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
E) CH3CH2C CCH3 + 2 H2 → CH3CH2CH2
CCH3 + 2 H2 → CH3CH2CH2  CH2CH3
CH2CH3
 CCH3 + 2 H2 → ?
CCH3 + 2 H2 → ?A) CH3CH2C
 CCH3 + 3 H2 → CH3CH2CH3 + CH3CH3
CCH3 + 3 H2 → CH3CH2CH3 + CH3CH3B) CH3CH2C
 CCH3 + 2 H2 → CH3CH2CH2CH2CH3
CCH3 + 2 H2 → CH3CH2CH2CH2CH3C) CH3CH2C
 CCH3 + 6 H2 → 5 CH4
CCH3 + 6 H2 → 5 CH4D) 2 CH3CH2C
 CCH3 + 2 H2 → CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
CCH3 + 2 H2 → CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3E) CH3CH2C
 CCH3 + 2 H2 → CH3CH2CH2
CCH3 + 2 H2 → CH3CH2CH2  CH2CH3
CH2CH3
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
41
Determine the products of the following reaction: 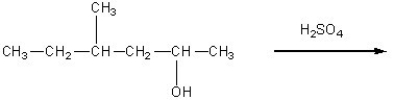
A)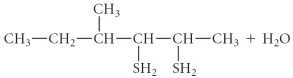
B)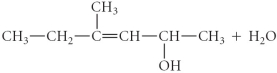
C)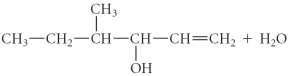
D)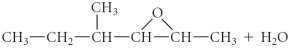
E)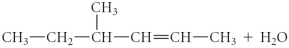

A)
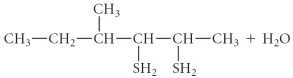
B)
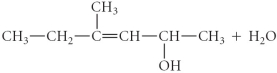
C)
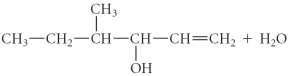
D)

E)
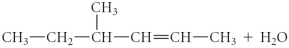

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
42
Arrange the following in order from most oxidized to least oxidized. 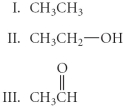
A) II > I > III
B) III > II > I
C) III > I > III
D) I > II > III
E) II > III > I
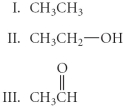
A) II > I > III
B) III > II > I
C) III > I > III
D) I > II > III
E) II > III > I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
43
Arrange the following in order from least oxidized to most oxidized. 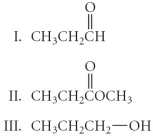
A) II < I < III
B) III < I = II
C) III < I < II
D) I < II < III
E) I < III < II

A) II < I < III
B) III < I = II
C) III < I < II
D) I < II < III
E) I < III < II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
44
Name the following compound. 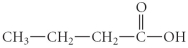
A) propyl butanoate
B) butanoic acid
C) 1-butanal
D) 1-butanoate
E) propyl methanoate
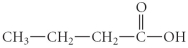
A) propyl butanoate
B) butanoic acid
C) 1-butanal
D) 1-butanoate
E) propyl methanoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
45
Give the organic product for the following reaction in the presence of sulfuric acid. CH3CH2COOH + CH3CH2CH2OH →
A) CH3CH2COOCH2CH3
B) CH3CH2COOCH2CH2CH3
C) CH3CH2CH2COOCH2CH3
D) CH3CH2COCH2CH2CH3
E) CH3CH2OCH2CH2CH3
A) CH3CH2COOCH2CH3
B) CH3CH2COOCH2CH2CH3
C) CH3CH2CH2COOCH2CH3
D) CH3CH2COCH2CH2CH3
E) CH3CH2OCH2CH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
46
Determine the products of the following reaction: 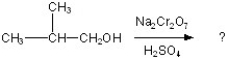
A)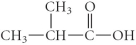
B)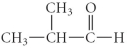
C)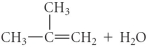
D)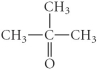
E)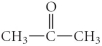

A)
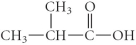
B)
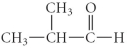
C)
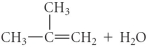
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
47
Give the product of the oxidation of the following compound. 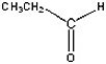
A) CH3CH2CHO
B) CH3CH2CH2OH
C) CH3CH2CH3
D) CH3CH2COOH
E) CH3CH2OCH3

A) CH3CH2CHO
B) CH3CH2CH2OH
C) CH3CH2CH3
D) CH3CH2COOH
E) CH3CH2OCH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
48
Give the product for the dehydration of CH3CH2CH2OH.
A) CH3CH2CH2OCH2CH2CH3
B) CH3CH=CH2
C) CH3CH=CHCH3
D) CH3CH2CH=CH2
E) CH2=C=CH2
A) CH3CH2CH2OCH2CH2CH3
B) CH3CH=CH2
C) CH3CH=CHCH3
D) CH3CH2CH=CH2
E) CH2=C=CH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
49
Determine the products of the following reaction: 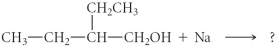
A)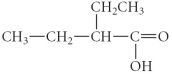
B)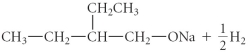
C)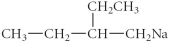
D)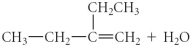
E)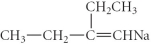
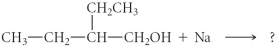
A)
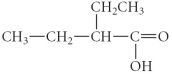
B)
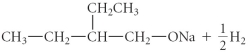
C)
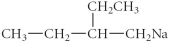
D)
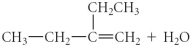
E)
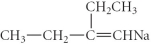

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
50
A carboxylic acid reacts with itself to form
A) an ether
B) an amine
C) an amide
D) an ester
E) an anhydride
A) an ether
B) an amine
C) an amide
D) an ester
E) an anhydride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
51
Determine the product(s)of the reduction of the following compound: 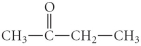
A)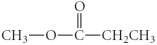
B)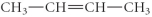
C)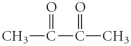
D)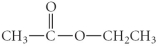
E)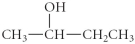
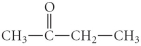
A)
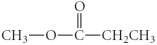
B)
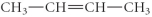
C)
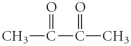
D)
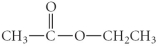
E)
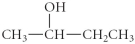

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which is an example of a ketone?
A) vanillin
B) propanal
C) cinnamaldehyde
D) ionone
E) formaldehyde
A) vanillin
B) propanal
C) cinnamaldehyde
D) ionone
E) formaldehyde

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
53
Give the product of the reduction of the following compound. 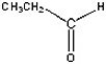
A) CH3CH2CHO
B) CH3CH2CH2OH
C) CH3CH2CH3
D) CH3CH2COOH
E) CH3CH2OCH3

A) CH3CH2CHO
B) CH3CH2CH2OH
C) CH3CH2CH3
D) CH3CH2COOH
E) CH3CH2OCH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
54
What compound is combined with yeast to form ethanol?
A) glucose
B) methanol
C) propanone
D) naphthalene
E) ethane
A) glucose
B) methanol
C) propanone
D) naphthalene
E) ethane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
55
What was commonly used as an anesthetic?
A) triethylamine
B) diethyl ether
C) ethyl butanoate
D) methanol
E) acetic acid
A) triethylamine
B) diethyl ether
C) ethyl butanoate
D) methanol
E) acetic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
56
Determine the products of the following reaction: 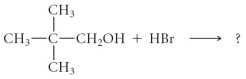
A)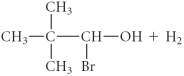
B)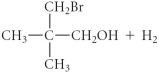
C)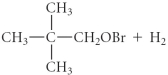
D)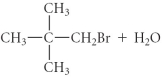
E)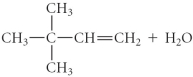
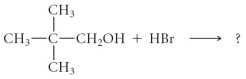
A)
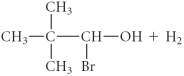
B)
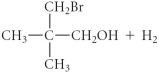
C)
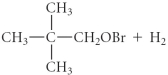
D)
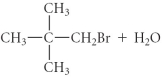
E)
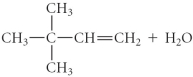

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
57
The difference between ethane and ethylamine is the presence of which element?
A) carbon
B) oxygen
C) hydrogen
D) nitrogen
E) sulfur
A) carbon
B) oxygen
C) hydrogen
D) nitrogen
E) sulfur

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
58
Determine the product(s)of the following reaction: 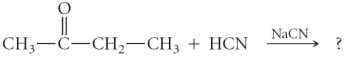
A)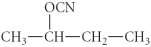
B)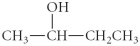
C)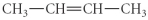
D)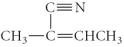
E)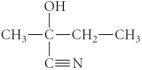
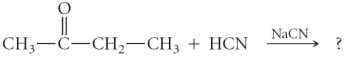
A)
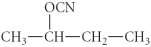
B)
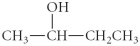
C)
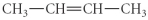
D)
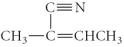
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
59
Identify the most oxidized compound.
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH3
D) CH3CH2OCH3
E) CH3CH2CH2OH
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH3
D) CH3CH2OCH3
E) CH3CH2CH2OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
60
A carboxylic acid reacts with an alcohol to form
A) an ether
B) an amine
C) an amide
D) an ester
E) an anhydride
A) an ether
B) an amine
C) an amide
D) an ester
E) an anhydride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
61
Give the organic product for the following reaction. CH3CH2COOH + CH3CH2CH2NH2 →
A) CH3CH2NHCH2CH2CH3
B) CH3CH2CON(CH2CH2CH3)2
C) CH3CH2CH2CONHCH2CH3
D) CH3CH2CONHCH2CH2CH3
E) CH3CH2N(CH2CH2CH3)2
A) CH3CH2NHCH2CH2CH3
B) CH3CH2CON(CH2CH2CH3)2
C) CH3CH2CH2CONHCH2CH3
D) CH3CH2CONHCH2CH2CH3
E) CH3CH2N(CH2CH2CH3)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
62
cadaverine and putrescine are categorized as:
A) esters
B) ketones
C) aldehydes
D) carboxylic acids
E) amines
A) esters
B) ketones
C) aldehydes
D) carboxylic acids
E) amines

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
63
Name the following compound. 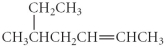
A) 2-ethyl-4- hexene
B) 4-isobutyl-2- butene
C) 3-methyl-5- heptane
D) 4-isopropyl-2- butene
E) 5-methyl-2-heptene
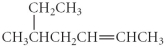
A) 2-ethyl-4- hexene
B) 4-isobutyl-2- butene
C) 3-methyl-5- heptane
D) 4-isopropyl-2- butene
E) 5-methyl-2-heptene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
64
Give the organic product for the following reaction. CH3CH2CH2NH2 + HCl →
A) ClCH2CH2CH2NH2
B) CH3CHClCH2NH2
C) CH3CH2CHClNH2
D) CH3CH2CH2NHCl
E) CH3CH2CH2NH3+ Cl-
A) ClCH2CH2CH2NH2
B) CH3CHClCH2NH2
C) CH3CH2CHClNH2
D) CH3CH2CH2NHCl
E) CH3CH2CH2NH3+ Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
65
How many isomers are there for C8H18 ?
A) 18
B) 19
C) 20
D) 21
A) 18
B) 19
C) 20
D) 21

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
66
Vanallin and cinnamaldehyde are categorized as:
A) esters
B) ketones
C) aldehydes
D) carboxylic acids
E) amines
A) esters
B) ketones
C) aldehydes
D) carboxylic acids
E) amines

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which of the following compounds exhibit optical isomerism?
A) sec-butylbenzene
B) CH2I2
C) CF2ClI
D) CHF2I
E) tert-butylbenzene
A) sec-butylbenzene
B) CH2I2
C) CF2ClI
D) CHF2I
E) tert-butylbenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which of the following names is correct?
A) 2-methyl-1-butene
B) 3,3- dipropyl-4- propene
C) 2-ethyl-3- butene
D) 1- ethyl-2-pentene
E) None of the above are correct.
A) 2-methyl-1-butene
B) 3,3- dipropyl-4- propene
C) 2-ethyl-3- butene
D) 1- ethyl-2-pentene
E) None of the above are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of the following compounds exhibits optical isomerism?
A) CH3-CH2-CH3
B) CH3-CH2-CHI-CH3
C) CH3-CHI-CH3
D) CH3-CH2-CH2I
E) CH3-CH2-CBr2-CH3
A) CH3-CH2-CH3
B) CH3-CH2-CHI-CH3
C) CH3-CHI-CH3
D) CH3-CH2-CH2I
E) CH3-CH2-CBr2-CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following alkane names is correct?
A) 2,3-dibutylpropane
B) 3-ethyl-4- methylbutane
C) 4-ethyl-5- butyl heptene
D) 3,3-dibutylpentane
E) None of the above names are correct.
A) 2,3-dibutylpropane
B) 3-ethyl-4- methylbutane
C) 4-ethyl-5- butyl heptene
D) 3,3-dibutylpentane
E) None of the above names are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
71
Name the following compound. 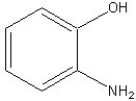
A) 2-aminophenol
B) 2-aminobenzaldehyde
C) 2-aminoaniline
D) 1-methylphenol
E) 2-hydroxybenzaldehyde

A) 2-aminophenol
B) 2-aminobenzaldehyde
C) 2-aminoaniline
D) 1-methylphenol
E) 2-hydroxybenzaldehyde

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
72
Name the following compound. 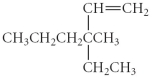
A) 2,2-diethylpentane
B) 2,2-diethyl1-hexene
C) 4-ethyl-4-methyl-5- hexane
D) 3-ethyl-3-methyl-1-hexene
E) 4-ethyl-4-methylhexane
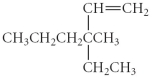
A) 2,2-diethylpentane
B) 2,2-diethyl1-hexene
C) 4-ethyl-4-methyl-5- hexane
D) 3-ethyl-3-methyl-1-hexene
E) 4-ethyl-4-methylhexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
73
Name the following compound. 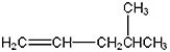
A) 2- methyl-4- pentane
B) 1,1-dimethyl-3- butene
C) 4-methyl-1-pentene
D) hexane
E) 2-methylpentane

A) 2- methyl-4- pentane
B) 1,1-dimethyl-3- butene
C) 4-methyl-1-pentene
D) hexane
E) 2-methylpentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following alkane names is correct?
A) 1,2-dimethylhexane
B) 1- propylnonane
C) 4,5-diethylpentane
D) 2-methyl-3- ethylhexane
E) All of the above names are correct.
A) 1,2-dimethylhexane
B) 1- propylnonane
C) 4,5-diethylpentane
D) 2-methyl-3- ethylhexane
E) All of the above names are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
75
Methyl butanoate and ethyl butanoate are categorized as:
A) esters
B) ketones
C) aldehydes
D) carboxylic acids
E) amines
A) esters
B) ketones
C) aldehydes
D) carboxylic acids
E) amines

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which compound is a saturated hydrocarbon?
A) bromobenzene
B) cyclohexene
C) 2, 4- dimethylpentane
D) 2-octene
A) bromobenzene
B) cyclohexene
C) 2, 4- dimethylpentane
D) 2-octene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
77
Name the following compound. 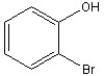
A) 2-bromophenol
B) 2-bromoaniline
C) 2-bromonaphthalene
D) 2-bromotoluene
E) 2-bromoanisole

A) 2-bromophenol
B) 2-bromoaniline
C) 2-bromonaphthalene
D) 2-bromotoluene
E) 2-bromoanisole

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
78
Name the following compound. 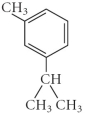
A) 1-methyl-3-isopropylhexene
B) 4-propyl-6-methylcyclohexene
C) 4-isopropyl-6-methylbenzene
D) 1-butylbenzene
E) 1-isopropyl-3-methylbenzene

A) 1-methyl-3-isopropylhexene
B) 4-propyl-6-methylcyclohexene
C) 4-isopropyl-6-methylbenzene
D) 1-butylbenzene
E) 1-isopropyl-3-methylbenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
79
All of the following compounds exhibit geometric isomerism EXCEPT
A) CFCl=CHBr
B) CH3ClC=CBrCH3
C) CHBr=CHCl
D) CH2=CH-CH3
E) All of the above exhibit geometric isomerism.
A) CFCl=CHBr
B) CH3ClC=CBrCH3
C) CHBr=CHCl
D) CH2=CH-CH3
E) All of the above exhibit geometric isomerism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck
80
Name the following compound. 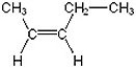
A) trans-2- butene
B) cis-2- butene
C) cis-2-pentene
D) trans- 2- pentane
E) 2- butane

A) trans-2- butene
B) cis-2- butene
C) cis-2-pentene
D) trans- 2- pentane
E) 2- butane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 105 في هذه المجموعة.
فتح الحزمة
k this deck








