Deck 6: Chemical Bonding I
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/139
العب
ملء الشاشة (f)
Deck 6: Chemical Bonding I
1
Identify the number of bonding pairs and lone pairs of electrons in water.
A) 1 bonding pair and 1 lone pair
B) 1 bonding pair and 2 lone pairs
C) 2 bonding pairs and 2 lone pairs
D) 2 bonding pairs and 1 lone pair
E) 3 bonding pairs and 2 lone pairs
A) 1 bonding pair and 1 lone pair
B) 1 bonding pair and 2 lone pairs
C) 2 bonding pairs and 2 lone pairs
D) 2 bonding pairs and 1 lone pair
E) 3 bonding pairs and 2 lone pairs
2 bonding pairs and 2 lone pairs
2
Using periodic trends,place the following bonds in order of increasing ionic character. Si-P Si-Cl Si-S
A) Si-P < Si-Cl < Si-S
B) Si-P < Si-S < Si-Cl
C) Si-S < Si-Cl < Si-P
D) Si-Cl < Si-P < Si-S
E) Si-Cl < Si-S < Si-P
A) Si-P < Si-Cl < Si-S
B) Si-P < Si-S < Si-Cl
C) Si-S < Si-Cl < Si-P
D) Si-Cl < Si-P < Si-S
E) Si-Cl < Si-S < Si-P
Si-P < Si-S < Si-Cl
3
Give the number of valence electrons for XeI2.
A) 22
B) 20
C) 18
D) 24
E) 16
A) 22
B) 20
C) 18
D) 24
E) 16
22
4
Choose the best Lewis structure for SF4.
A)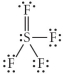
B)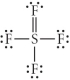
C)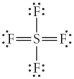
D)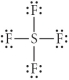
E)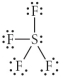
A)
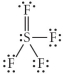
B)
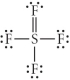
C)
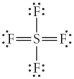
D)
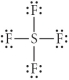
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
5
Choose the bond below that is LEAST polar.
A) P-F
B) C-Br
C) C-F
D) C-I
E) C-Cl
A) P-F
B) C-Br
C) C-F
D) C-I
E) C-Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
6
Identify the compound with the smallest dipole moment in the gas phase.
A) Cl2
B) ClF
C) HF
D) LiF
A) Cl2
B) ClF
C) HF
D) LiF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
7
Give the number of valence electrons for CH2Cl2.
A) 16
B) 18
C) 20
D) 22
E) 12
A) 16
B) 18
C) 20
D) 22
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
8
Choose the best Lewis structure for CH2Cl2.
A)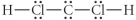
B)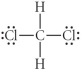
C)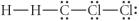
D)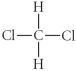
E)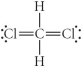
A)
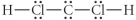
B)

C)
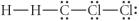
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
9
Identify the compound with the highest percent ionic character.
A) HF
B) IBr
C) HCl
D) LiF
A) HF
B) IBr
C) HCl
D) LiF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
10
Identify the shortest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same length.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same length.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
11
Identify the compound with the largest dipole moment in the gas phase.
A) Cl2
B) ClF
C) HF
D) LiF
A) Cl2
B) ClF
C) HF
D) LiF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
12
Choose the best Lewis structure for ICl5.
A)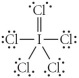
B)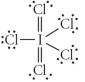
C)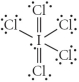
D)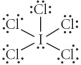
E)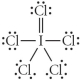
A)
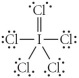
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
13
Identify the compound with the smallest percent ionic character.
A) HF
B) IBr
C) HCl
D) LiF
A) HF
B) IBr
C) HCl
D) LiF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
14
Choose the best Lewis structure for BeF2.
A)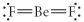
B)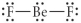
C)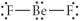
D)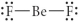
E)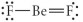
A)
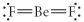
B)
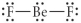
C)
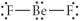
D)
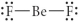
E)
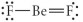

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
15
Choose the best Lewis structure for XeI2.
A)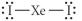
B)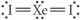
C)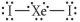
D)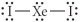
E)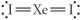
A)
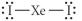
B)
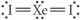
C)
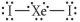
D)
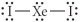
E)
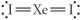

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
16
Give the number of valence electrons for ICl5.
A) 36
B) 40
C) 42
D) 44
E) 46
A) 36
B) 40
C) 42
D) 44
E) 46

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
17
Identify the weakest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
18
Identify the strongest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same strength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
19
Choose the best Lewis structure for OCl2.
A)
B)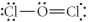
C)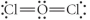
D)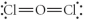
E)
A)

B)
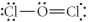
C)
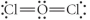
D)
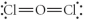
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
20
Choose the bond below that is MOST polar.
A) H-I
B) H-Br
C) H-F
D) H-Cl
E) C-H
A) H-I
B) H-Br
C) H-F
D) H-Cl
E) C-H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
21
Choose the best Lewis structure for PO43⁻.
A)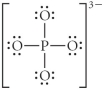
B)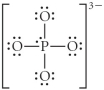
C)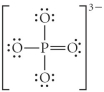
D)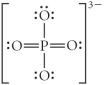
E)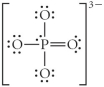
A)

B)

C)

D)

E)
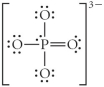

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
22
Draw the best Lewis structure for CH3+1.What is the formal charge on the C?
A) 0
B) 1
C) -1
D) 2
A) 0
B) 1
C) -1
D) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
23
Draw the Lewis structure for NO2⁻ including any valid resonance structures.Which of the following statements is TRUE?
A) The nitrite ion contains one N-O single bond and one N O double bond.
O double bond.
B) The nitrite ion contains two N-O bonds that are equivalent to 1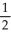 bonds.
bonds.
C) The nitrite ion contains two N O double bonds.
O double bonds.
D) The nitrite ion contains two N-O single bonds.
E) None of the above are true.
A) The nitrite ion contains one N-O single bond and one N
 O double bond.
O double bond.B) The nitrite ion contains two N-O bonds that are equivalent to 1
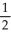 bonds.
bonds.C) The nitrite ion contains two N
 O double bonds.
O double bonds.D) The nitrite ion contains two N-O single bonds.
E) None of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
24
Give the number of valence electrons for SO42-.
A) 32
B) 30
C) 34
D) 28
E) 36
A) 32
B) 30
C) 34
D) 28
E) 36

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
25
Choose the best Lewis structure for SeO42⁻.
A)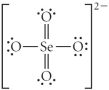
B)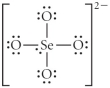
C)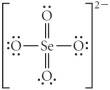
D)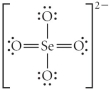
E)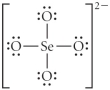
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following resonance structures for OCN⁻ will contribute most to the correct structure of OCN⁻?
A) O(2 lone pairs) C
C  N (2 lone pairs)
N (2 lone pairs)
B) O(1 lone pair) C-N(3 lone pairs)
C-N(3 lone pairs)
C) O(1 lone pair) C(2 lone pairs)
C(2 lone pairs)  N(1 lone pair)
N(1 lone pair)
D) O(3 lone pairs)-C N(with 1 lone pair)
N(with 1 lone pair)
E) They all contribute equally to the correct structure of OCN⁻.
A) O(2 lone pairs)
 C
C  N (2 lone pairs)
N (2 lone pairs)B) O(1 lone pair)
 C-N(3 lone pairs)
C-N(3 lone pairs)C) O(1 lone pair)
 C(2 lone pairs)
C(2 lone pairs)  N(1 lone pair)
N(1 lone pair)D) O(3 lone pairs)-C
 N(with 1 lone pair)
N(with 1 lone pair)E) They all contribute equally to the correct structure of OCN⁻.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
27
Draw the Lewis structure for CO32- including any valid resonance structures.Which of the following statements is TRUE?
A) The CO32- ion contains one C-O single bond and two C O double bonds.
O double bonds.
B) The CO32- ion contains two C-O single bonds and one C O double bond.
O double bond.
C) The CO32- ion contains three C-O double bonds.
D) The CO32- ion contains two C-O single bonds and one C O triple bond.
O triple bond.
E) None of the above are true.
A) The CO32- ion contains one C-O single bond and two C
 O double bonds.
O double bonds.B) The CO32- ion contains two C-O single bonds and one C
 O double bond.
O double bond.C) The CO32- ion contains three C-O double bonds.
D) The CO32- ion contains two C-O single bonds and one C
 O triple bond.
O triple bond.E) None of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
28
Using Lewis structures and formal charge,which of the following ions is most stable?
OCN⁻ ONC⁻ NOC⁻
A) OCN⁻
B) ONC⁻
C) NOC⁻
D) None of these ions are stable according to Lewis theory.
E) All of these compounds are equally stable according to Lewis theory.
OCN⁻ ONC⁻ NOC⁻
A) OCN⁻
B) ONC⁻
C) NOC⁻
D) None of these ions are stable according to Lewis theory.
E) All of these compounds are equally stable according to Lewis theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
29
Identify the compound with atoms that have an incomplete octet.
A) ICl5
B) CO2
C) BF3
D) Cl2
E) CO
A) ICl5
B) CO2
C) BF3
D) Cl2
E) CO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
30
Choose the best Lewis structure for NH4⁺.
A)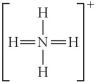
B)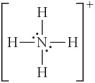
C)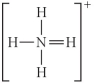
D)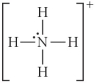
E)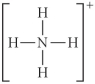
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which compound has the longest carbon-carbon bond length?
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
32
Choose the best Lewis structure for NO3⁻.
A)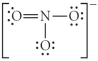
B)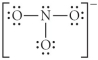
C)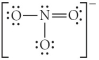
D)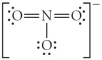
E)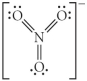
A)
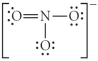
B)
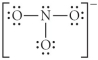
C)
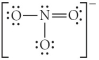
D)
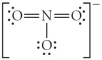
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
33
Draw the best Lewis structure for the free radical,NO2.What is the formal charge on the N?
A) 0
B) +1
C) -1
D) +2
E) -2
A) 0
B) +1
C) -1
D) +2
E) -2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which compound has the shortest carbon-carbon bond length?
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond lengths are the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
35
Draw the best Lewis structure for BrO4⁻ and determine the formal charge on bromine.
A) -1
B) +1
C) 0
D) +2
E) +3
A) -1
B) +1
C) 0
D) +2
E) +3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
36
Draw the best Lewis structure for Cl3⁻.What is the formal charge on the central Cl atom?
A) -1
B) 0
C) +1
D) +2
E) -2
A) -1
B) 0
C) +1
D) +2
E) -2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
37
Draw the best Lewis structure for CH3-1.What is the formal charge on the C?
A) 0
B) 1
C) -1
D) 2
A) 0
B) 1
C) -1
D) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
38
Draw the Lewis structure for SO42⁻.How many equivalent resonance structures can be drawn?
A) 6
B) 2
C) 4
D) 3
E) 8
A) 6
B) 2
C) 4
D) 3
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
39
Choose the best Lewis structure for BF3.
A)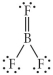
B)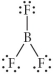
C)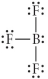
D)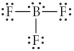
E)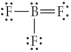
A)
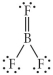
B)

C)
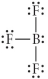
D)
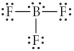
E)
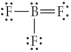

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
40
Choose the best Lewis structure for SO42⁻.
A)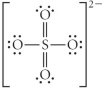
B)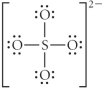
C)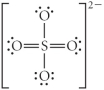
D)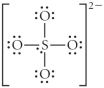
E)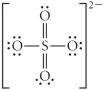
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
41
Determine the electron geometry (eg)and molecular geometry (mg)of XeF2.
A) eg = trigonal bipyramidal, mg = bent
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = linear
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent
A) eg = trigonal bipyramidal, mg = bent
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = linear
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
42
Determine the electron geometry (eg)and molecular geometry (mg)of CO32⁻.
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
43
Place the following in order of increasing bond length. C-F C-S C-Cl
A) C-S < C-Cl < C-F
B) C-Cl < C-F < C-S
C) C-F < C-S < C-Cl
D) C-F < C-Cl < C-S
E) C-S < C-F < C-Cl
A) C-S < C-Cl < C-F
B) C-Cl < C-F < C-S
C) C-F < C-S < C-Cl
D) C-F < C-Cl < C-S
E) C-S < C-F < C-Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which compound has the highest carbon-carbon bond strength?
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond strengths are the same.
A) CH3CH3
B) CH2CH2
C) HCCH
D) All bond strengths are the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
45
Determine the electron geometry (eg)and molecular geometry (mg)of ICl2⁻.
A) eg = tetrahedral, mg = bent
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = trigonal planar
E) eg = octahedral, mg = linear
A) eg = tetrahedral, mg = bent
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = trigonal planar
E) eg = octahedral, mg = linear

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
46
The bond angle in NH3 is
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
47
Place the following in order of decreasing XO bond length,where "X" represents the central atom in each of the following compounds or ions. SiO32⁻ CO2 CO32⁻
A) CO2 > SiO32⁻ > CO32⁻
B) CO2 > CO32⁻ > SiO32⁻
C) CO32⁻ > CO2 > SiO32⁻
D) CO32⁻ > SiO32⁻ > CO2
E) SiO32⁻ > CO32⁻ > CO2
A) CO2 > SiO32⁻ > CO32⁻
B) CO2 > CO32⁻ > SiO32⁻
C) CO32⁻ > CO2 > SiO32⁻
D) CO32⁻ > SiO32⁻ > CO2
E) SiO32⁻ > CO32⁻ > CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
48
Determine the electron geometry (eg)and molecular geometry (mg)of PF5.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = octahedral
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = trigonal pyramidal
E) eg = trigonal planar, mg = octahedral
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = octahedral
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = trigonal pyramidal
E) eg = trigonal planar, mg = octahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
49
Determine the electron geometry (eg)and molecular geometry (mg)of CO2.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = trigonal planar, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = trigonal planar, mg = trigonal planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
50
Determine the electron geometry (eg)and molecular geometry (mg)of NCl3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = tetrahedral, mg = trigonal pyramidal
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = tetrahedral, mg = trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
51
Determine the electron geometry (eg)and molecular geometry (mg)of CH3+1.
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal planar, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
52
Determine the electron geometry (eg)and molecular geometry (mg)of Br F3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = trigonal bipyramidal, mg = T-shape
C) eg = trigonal planar, mg = bent
D) eg = trigonal bipyramidal, mg = see-saw
E) eg = tetrahedral, mg = trigonal pyramidal
A) eg = trigonal planar, mg = trigonal planar
B) eg = trigonal bipyramidal, mg = T-shape
C) eg = trigonal planar, mg = bent
D) eg = trigonal bipyramidal, mg = see-saw
E) eg = tetrahedral, mg = trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
53
Identify the bond with the highest bond energy.
A) Si = O
B) N = N
C) C = C
D) C = N
E) O = O
A) Si = O
B) N = N
C) C = C
D) C = N
E) O = O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
54
Place the following in order of increasing bond length. NO2⁻ NO3⁻ NO
A) NO < NO2⁻ < NO3⁻
B) NO2⁻ < NO3⁻ < NO
C) NO3⁻ < NO < NO2⁻
D) NO < NO3⁻ < NO2⁻
E) NO3⁻ < NO2⁻ < NO
A) NO < NO2⁻ < NO3⁻
B) NO2⁻ < NO3⁻ < NO
C) NO3⁻ < NO < NO2⁻
D) NO < NO3⁻ < NO2⁻
E) NO3⁻ < NO2⁻ < NO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
55
Determine the electron geometry (eg)and molecular geometry (mg)of SiF4.
A) eg = tetrahedral, mg = trigonal pyramidal
B) eg = octahedral, mg = square planar
C) eg = trigonal bipyramidal, mg = trigonal pyramidal
D) eg = tetrahedral, mg = bent
E) eg = tetrahedral, mg = tetrahedral
A) eg = tetrahedral, mg = trigonal pyramidal
B) eg = octahedral, mg = square planar
C) eg = trigonal bipyramidal, mg = trigonal pyramidal
D) eg = tetrahedral, mg = bent
E) eg = tetrahedral, mg = tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
56
Determine the electron geometry (eg)and molecular geometry(mg)of BCl3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = bent
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = bent
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
57
Give the approximate bond angle for a molecule with an octahedral shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
58
The bond angle in H2O is
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°
A) 107°
B) 104.5°
C) 120°
D) 109.5°
E) 95°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
59
Rank the following molecules in decreasing bond energy. Cl2 Br2 F2 I2
A) I2 > Br2 > Cl2 > F2
B) Cl2 > Br2 > F2 > I2
C) I2 > Cl2 > Br2 > F2
D) Cl2 > I2 > F2 > Br2
A) I2 > Br2 > Cl2 > F2
B) Cl2 > Br2 > F2 > I2
C) I2 > Cl2 > Br2 > F2
D) Cl2 > I2 > F2 > Br2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
60
Place the following in order of decreasing bond length. H-F H-I H-Br
A) H-F > H-Br > H-I
B) H-I > H-F > H-Br
C) H-I > H-Br > H-F
D) H-Br > H-F > H-I
E) H-F > H-I > H-Br
A) H-F > H-Br > H-I
B) H-I > H-F > H-Br
C) H-I > H-Br > H-F
D) H-Br > H-F > H-I
E) H-F > H-I > H-Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
61
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom CH3OCH3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
62
Determine the electron geometry (eg),molecular geometry (mg),and polarity of SO2.
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = bent, polar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = tetrahedral, nonpolar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = bent, polar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = tetrahedral, nonpolar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
63
Place the following in order of increasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. HCN H2O H3O⁺
A) HCN < H2O < H3O⁺
B) H3O⁺ < H2O < HCN
C) HCN < H3O⁺ < H2O
D) H2O < HCN < H3O⁺
E) H2O < H3O⁺ < HCN
A) HCN < H2O < H3O⁺
B) H3O⁺ < H2O < HCN
C) HCN < H3O⁺ < H2O
D) H2O < HCN < H3O⁺
E) H2O < H3O⁺ < HCN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
64
Determine the electron geometry (eg)and molecular geometry (mg)of XeF4.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
65
Place the following in order of increasing dipole moment. I.BCl3 II.BIF2 III.BClF2
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
66
Determine the electron geometry,molecular geometry and polarity of SF6.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal, nonpolar
B) eg = tetrahedral, mg = tetrahedral, polar
C) eg = trigonal bipyramidal, mg = see-saw, polar
D) eg = octahedral, mg = trigonal bipyramidal, nonpolar
E) eg = octahedral, mg = octahedral, nonpolar
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal, nonpolar
B) eg = tetrahedral, mg = tetrahedral, polar
C) eg = trigonal bipyramidal, mg = see-saw, polar
D) eg = octahedral, mg = trigonal bipyramidal, nonpolar
E) eg = octahedral, mg = octahedral, nonpolar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
67
Determine the electron geometry (eg),molecular geometry (mg),and polarity of SO3.
A) eg = tetrahedral, mg = trigonal pyramidal, polar
B) eg = tetrahedral, mg = tetrahedral, nonpolar
C) eg = trigonal planar, mg = trigonal planar, nonpolar
D) eg = trigonal bipyramidal, mg = trigonal planar, polar
E) eg = trigonal pyramidal, mg = bent, nonpolar
A) eg = tetrahedral, mg = trigonal pyramidal, polar
B) eg = tetrahedral, mg = tetrahedral, nonpolar
C) eg = trigonal planar, mg = trigonal planar, nonpolar
D) eg = trigonal bipyramidal, mg = trigonal planar, polar
E) eg = trigonal pyramidal, mg = bent, nonpolar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
68
Determine the electron geometry,molecular geometry and polarity of HBrO2.
A) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
B) eg = octahedral, mg = square planar, nonpolar
C) eg = tetrahedral, mg = bent, polar
D) eg = tetrahedral, mg = linear, nonpolar
E) eg = linear, mg = linear, polar
A) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
B) eg = octahedral, mg = square planar, nonpolar
C) eg = tetrahedral, mg = bent, polar
D) eg = tetrahedral, mg = linear, nonpolar
E) eg = linear, mg = linear, polar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
69
Determine the electron geometry (eg),molecular geometry (mg),and polarity of PCl3.
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = trigonal planar, nonpolar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = trigonal pyramidal, polar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
A) eg = tetrahedral, mg = bent, polar
B) eg = trigonal planar, mg = trigonal planar, nonpolar
C) eg = linear, mg = linear, nonpolar
D) eg = tetrahedral, mg = trigonal pyramidal, polar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
70
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom H2CO.
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal planar, eg = trigonal planar
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal planar, eg = trigonal planar
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
71
How many of the following molecules are polar?
PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4
PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
72
Consider the molecule below.Determine the molecular geometry at each of the 3 labeled atoms. 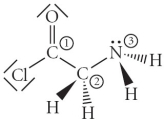
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal

A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
73
Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 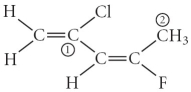
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2 = bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw

A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2 = bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
74
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. CS2 CF4 SCl2
A) CS2 > SCl2 > CF4
B) SCl2 > CF4 > CS2
C) CF4 > SCl2 > CS2
D) CS2 > CF4 > SCl2
E) CF4 > CS2 > SCl2
A) CS2 > SCl2 > CF4
B) SCl2 > CF4 > CS2
C) CF4 > SCl2 > CS2
D) CS2 > CF4 > SCl2
E) CF4 > CS2 > SCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
75
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. N2O NCl3 NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
76
Place the following in order of increasing F-A-F bond angle,where A represents the central atom in each molecule. PF3 OF2 PF4⁺
A) PF3 < OF2 < PF4⁺
B) OF2 < PF3 < PF4⁺
C) OF2 < PF4⁺ < PF3
D) PF4⁺ < OF2 < PF3
E) PF4⁺ < PF3 < OF2
A) PF3 < OF2 < PF4⁺
B) OF2 < PF3 < PF4⁺
C) OF2 < PF4⁺ < PF3
D) PF4⁺ < OF2 < PF3
E) PF4⁺ < PF3 < OF2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
77
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom CH3OCH3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
78
Determine the electron geometry (eg),molecular geometry(mg)and polarity of XeO3.
A) eg = trigonal planar, mg = trigonal planar, nonpolar
B) eg = tetrahedral, mg = trigonal pyramidal, polar
C) eg = trigonal planar, mg = trigonal pyramidal, polar
D) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
E) eg = octahedral, mg = tetrahedral, nonpolar
A) eg = trigonal planar, mg = trigonal planar, nonpolar
B) eg = tetrahedral, mg = trigonal pyramidal, polar
C) eg = trigonal planar, mg = trigonal pyramidal, polar
D) eg = trigonal bipyramidal, mg = trigonal planar, nonpolar
E) eg = octahedral, mg = tetrahedral, nonpolar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
79
Place the following in order of increasing X-Se-X bond angle,where X represents the outer atoms in each molecule. SeO2 SeCl6 SeF2
A) SeCl6 < SeF2 < SeO2
B) SeF2 < SeO2 < SeCl6
C) SeF2 < SeCl6 < SeO2
D) SeO2 < SeF2 < SeCl6
E) SeCl6 < SeO2 < SeF2
A) SeCl6 < SeF2 < SeO2
B) SeF2 < SeO2 < SeCl6
C) SeF2 < SeCl6 < SeO2
D) SeO2 < SeF2 < SeCl6
E) SeCl6 < SeO2 < SeF2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
80
How many of the following molecules are polar?
BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0
BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck








