Deck 2: Chemical Measurements
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/21
العب
ملء الشاشة (f)
Deck 2: Chemical Measurements
1
The recommended daily allowance of calcium for men between the ages of 19 to 50 is 1 000 mg Ca.Three multivitamin tablets are analyzed for calcium gravimetrically with the precipitation of calcium cation by oxalate anion.If the mass dry calcium oxalate obtained is 2.0136 g,how many tablets must a man take in a given day to meet the recommended daily allowance?
Ca2+ (aq)+ C2O42− (aq) CaC2O4 (s)
Ca2+ (aq)+ C2O42− (aq) CaC2O4 (s)
five vitamin tablets
2
The planet Mars orbits 2.279 *1011 m from the Sun.Express the distance using the appropriate prefix.
A)227.9 Gm
B)227.9 mM
C)2.279 km
D)22.79 nm
E)None of these is correct.
A)227.9 Gm
B)227.9 mM
C)2.279 km
D)22.79 nm
E)None of these is correct.
227.9 Gm
3
A student must prepare 500.0 mL of solution containing 0.999 grams of solid copper(II)sulfate.Which of the following statements are FALSE regarding the proper procedure to prepare this solution?
I The 0.999 grams of solid copper(II)sulfate is dissolved in a 500.0-mL volumetric flask containing 500.0 mL of water.
II The 0.999 grams of solid copper(II)sulfate is dissolved in a 500.0-mL volumetric flask containing 400 mL of distilled water before dilution to 500.0-mL.
III The 0.999 grams of solid copper(II)sulfate is placed in an empty 500.0-mL volumetric flask,diluted to 500.0 mL and allowed to dissolve.
A)I and II
B)II and III
C)I and III
D)I,II,and III
E)None are false.
I The 0.999 grams of solid copper(II)sulfate is dissolved in a 500.0-mL volumetric flask containing 500.0 mL of water.
II The 0.999 grams of solid copper(II)sulfate is dissolved in a 500.0-mL volumetric flask containing 400 mL of distilled water before dilution to 500.0-mL.
III The 0.999 grams of solid copper(II)sulfate is placed in an empty 500.0-mL volumetric flask,diluted to 500.0 mL and allowed to dissolve.
A)I and II
B)II and III
C)I and III
D)I,II,and III
E)None are false.
I and III
4
Which of the statements below are TRUE for expressing the concentration of a 54.9-ppm Fe solution in terms of molarity?
I Iron's molar mass must be known to calculate the moles iron in solution.
II Iron's density must be known to calculate the mass iron in solution.
III The solution density must be known to calculate the solution volume.
IV The type of glassware used to prepare the solution must be known.
A)I,III,and IV
B)I and II
C)I and III
D)II and III
E)None of these is true.
I Iron's molar mass must be known to calculate the moles iron in solution.
II Iron's density must be known to calculate the mass iron in solution.
III The solution density must be known to calculate the solution volume.
IV The type of glassware used to prepare the solution must be known.
A)I,III,and IV
B)I and II
C)I and III
D)II and III
E)None of these is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
5
A mixture of 50.00 grams propane,C3H8,and 100.00 grams oxygen,O2,is combusted.____________________is the limiting reactant and____________________ grams of____________________ is in excess.(C3H8 = 44.10 g/mol) C3H8 (g)+ 5 O2 (g) 3 CO2 (g)+ 4 H2O (g)
A)Propane;22.44;oxygen
B)Oxygen;63.71;propane
C)Oxygen;22.42;propane
D)Propane;27.44;oxygen
E)None of these answers is correct.
A)Propane;22.44;oxygen
B)Oxygen;63.71;propane
C)Oxygen;22.42;propane
D)Propane;27.44;oxygen
E)None of these answers is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
6
The sulfur content of an ore is determined gravimetrically by reacting the ore with concentrated nitric acid and potassium chlorate,converting all sulfur to sulfate.The excess nitrate and chlorate is removed by reaction with concentrated hydrochloric acid and the sulfate is precipitated using barium cation. Ba2+ (aq)+ SO42- (aq) BaSO4 (s)
Analysis of 10.1830 grams of a sulfur containing ore yielded 13.0221 grams of BaSO4.What is the percent by mass sulfur in the ore? (BaSO4 = 233.43 g/mol)
A)32.18%
B)52.63%
C)10.74%
D)17.57%
E)The answer cannot be calculated with available data.
Analysis of 10.1830 grams of a sulfur containing ore yielded 13.0221 grams of BaSO4.What is the percent by mass sulfur in the ore? (BaSO4 = 233.43 g/mol)
A)32.18%
B)52.63%
C)10.74%
D)17.57%
E)The answer cannot be calculated with available data.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
7
Calculate the mass of Na2CO3 needed to prepare a 15.00 mM solution with a volume of 500.0 mL.(Na2CO3 = 105.9888 g/mol)
A)1)258 g
B)3)180 g
C)0)7949 g
D)7)076 g
E)0)0141 g
A)1)258 g
B)3)180 g
C)0)7949 g
D)7)076 g
E)0)0141 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
8
What volume of a 50.0% by mass NaOH solution must be diluted to prepare a 0.3500 M NaOH solution with a volume of 500 mL? The density of 50% NaOH solution is 1.515 g/mL at 25°C.(NaOH = 40.00 g/mol)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
9
The calorie content of a candy bar is 230 calories per serving (1 bar).Calculate the specific energy (kJ/g)of the candy bar.(1 candy bar = 52.7 g,1 calorie = 4.184 J)
A)2.90 *103 kJ/g
B)18.3 kJ/g
C)1.83 * 10−2 kJ/g
D)5.07* 104 kJ/g
E)9.59* 10−1 kJ/g
A)2.90 *103 kJ/g
B)18.3 kJ/g
C)1.83 * 10−2 kJ/g
D)5.07* 104 kJ/g
E)9.59* 10−1 kJ/g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
10
Calculate the molarity of a 30.0% hydrogen peroxide solution.The reported density of 30% hydrogen peroxide is 1.135 g/cm3.(H2O2 = 34.0147 g/mol)
A)7)77 M
B)0)0100 M
C)0)100 M
D)10.0 M
E)8)82 M
A)7)77 M
B)0)0100 M
C)0)100 M
D)10.0 M
E)8)82 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
11
What volume of 12.1 M HCl must be diluted to prepare a 0.2500 M HCl solution with a volume of 2.000 L?
A)41.3 mL
B)96.8 mL
C)10.3 mL
D)24.2 mL
E)6)05 mL
A)41.3 mL
B)96.8 mL
C)10.3 mL
D)24.2 mL
E)6)05 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
12
The maximum contaminant level for arsenic is 0.010 ppm for drinking water per EPA regulation.The arsenic concentration for the drinking water of a municipality was measured to be 4.92 * 10−6 M arsenic.What is the arsenic concentration of the water sample in ppm? Does the water sample meet EPA guidelines? Assume the drinking water sample has a density of 1.0000 g/mL.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
13
On average one gallon of kerosene contains 135,000 BTUs of heat energy per gallon combusted.Convert the energy content of kerosene to SI units.(1 BTU = 1055 J)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
14
An NaCl solution is labeled as having a concentration of 33.5% by mass.If the NaCl solution has a density of 1.0492 g/mL,what is the molarity of the solution? (NaCl= 58.44 g/mol)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
15
Calculate the mass NaCH3CO2 contained in 500.0 mL of a 0.1500 M NaCH3CO2 solution.(NaCH3CO2 = 82.0343 g/mol)
A)914.3 µg
B)283.4 g
C)24.61 µg
D)6)378 g
E)24.61 g
A)914.3 µg
B)283.4 g
C)24.61 µg
D)6)378 g
E)24.61 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
16
What volume of a 36% HCl solution must be diluted to prepare 1.000 L of a 0.1000 M HCl solution? The density of 36% HCl is 1.18 g/mL.
A)11.7 mL
B)8)6 mL
C)1)2 mL
D)10.1 mL
E)64.6 mL
A)11.7 mL
B)8)6 mL
C)1)2 mL
D)10.1 mL
E)64.6 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
17
A satellite in low Earth orbit with a circular orbit has an orbital speed of 7.3 km/s relative to the Earth's surface.Calculate the satellite's speed in miles per hour.
A)1.6 * 104 mile per hour
B)1.3 * 10−3 mile per hour
C)4.2 *104 mile per hour
D)3.3 *10−3 mile per hour
E)3.1 * 102 mile per hour
A)1.6 * 104 mile per hour
B)1.3 * 10−3 mile per hour
C)4.2 *104 mile per hour
D)3.3 *10−3 mile per hour
E)3.1 * 102 mile per hour

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
18
The gas mileage for a new car model destined for sale in Europe must be determined for regulatory and promotional purposes.If the car uses 10.5 gallons to travel 250 miles,what is the gas mileage in km/L?
A)174 km/L
B)3)25 km/L
C)67.3 km/L
D)14.9 km/L
E)10.1 km/L
A)174 km/L
B)3)25 km/L
C)67.3 km/L
D)14.9 km/L
E)10.1 km/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
19
When solutions of Pb2+ and CrO42− are mixed the precipitate PbCrO4 is produced.What volume of 0.1750 M CrO42− removes all lead from 50.00 mL of a 0.3400 M Pb2+ solution?
A)97.14 mL
B)25.74 mL
C)48.57 mL
D)194.29 mL
E)75.00 mL
A)97.14 mL
B)25.74 mL
C)48.57 mL
D)194.29 mL
E)75.00 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
20
Arrange the four microscopic views below of four different solutions in order of increasing concentration. 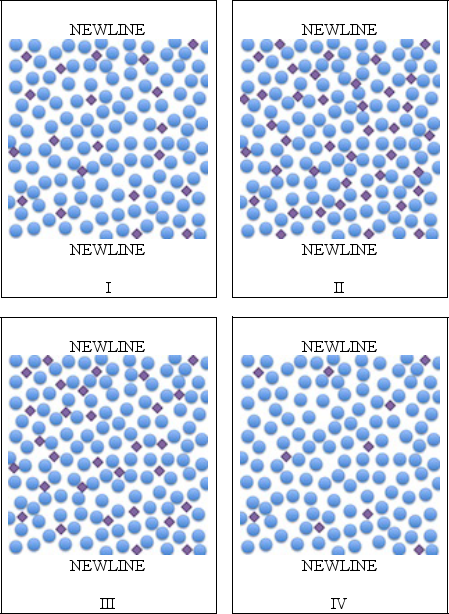
A)I,II,III,and IV
B)II,III,I,and IV
C)IV,II,III,and I
D)IV,I,III,and II
E)III,IV,II,and I

A)I,II,III,and IV
B)II,III,I,and IV
C)IV,II,III,and I
D)IV,I,III,and II
E)III,IV,II,and I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck
21
Aqueous lead precipitates when mixed with aqueous carbonate.
Pb2+ (aq)+ CO32− (aq) PbCO3 (s)
If 5.000 g Pb(NO3)2 and 2.500 g Na2CO3 are mixed in water,which ion is the limiting reactant? What mass PbCO3 is precipitated? (PbCO3 = 267.21 g/mol,Pb(NO3)2 = 331.2 g/mol,Na2CO3 = 105.9888 g/mol)
Pb2+ (aq)+ CO32− (aq) PbCO3 (s)
If 5.000 g Pb(NO3)2 and 2.500 g Na2CO3 are mixed in water,which ion is the limiting reactant? What mass PbCO3 is precipitated? (PbCO3 = 267.21 g/mol,Pb(NO3)2 = 331.2 g/mol,Na2CO3 = 105.9888 g/mol)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 21 في هذه المجموعة.
فتح الحزمة
k this deck








