Deck 7: Chemical Equilibrium
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/20
العب
ملء الشاشة (f)
Deck 7: Chemical Equilibrium
1
When the direction for a reaction is reversed the new value of K is:
A)K' = log(K).
B)K' = ln(K).
C)K' = 10K.
D)K' = 1/K.
E)K'= eK.
A)K' = log(K).
B)K' = ln(K).
C)K' = 10K.
D)K' = 1/K.
E)K'= eK.
K' = 1/K.
2
In which solution is solubility of silver chloride the largest?
A)Pure water
B)0)05 M CaCl2
C)0)10 M NaCl
D)0)10 M FeCl3
E)0)15 M AgNO3
A)Pure water
B)0)05 M CaCl2
C)0)10 M NaCl
D)0)10 M FeCl3
E)0)15 M AgNO3
Pure water
3
For the reaction aA (aq)+ bB (s) cC (l)+ dD (g),all of the statements below are TRUE,except:
A)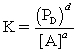
B)concentrations of solutes are expressed in moles per liter.
C)concentrations of gases are expressed in bars.
D)concentrations of pure solids,pure liquids and solvents are omitted because they are negligible.
E)quantities in the equilibrium expression are the ratio of the concentration of the species to the concentration in its standard state.
A)
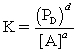
B)concentrations of solutes are expressed in moles per liter.
C)concentrations of gases are expressed in bars.
D)concentrations of pure solids,pure liquids and solvents are omitted because they are negligible.
E)quantities in the equilibrium expression are the ratio of the concentration of the species to the concentration in its standard state.
concentrations of pure solids,pure liquids and solvents are omitted because they are negligible.
4
Nickel forms three complexes with hydroxide,NiOH+ (aq),Ni(OH)2 (aq)and Ni(OH (aq).Which statement(s)regarding the impact of complex formation on the solubility of nickel hydroxide are FALSE?
I Hydroxide behaves as a Lewis acid when forming a complex with nickel.
II The total nickel concentration is [Ni2+]tot = [Ni2+] + [NiOH+] + [Ni(OH)2] + [Ni(OH].
III As [OH-] increases,the solubility of Ni(OH)2 initially decreases but then increases.
IV![<strong>Nickel forms three complexes with hydroxide,NiOH<sup>+</sup> (aq),Ni(OH)<sub>2</sub> (aq)and Ni(OH (aq).Which statement(s)regarding the impact of complex formation on the solubility of nickel hydroxide are FALSE? I Hydroxide behaves as a Lewis acid when forming a complex with nickel. II The total nickel concentration is [Ni<sup>2+</sup>]<sub>tot</sub> = [Ni<sup>2+</sup>] + [NiOH<sup>+</sup>] + [Ni(OH)<sub>2</sub>] + [Ni(OH]. III As [OH<sup>-</sup>] increases,the solubility of Ni(OH)<sub>2</sub> initially decreases but then increases. IV </strong> A)Only I B)II and III C)Only III D)Only IV E)I,II,III,and IV](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a3_fc33_a4b1_d3ab1238e74f_TB4000_11.jpg)
A)Only I
B)II and III
C)Only III
D)Only IV
E)I,II,III,and IV
I Hydroxide behaves as a Lewis acid when forming a complex with nickel.
II The total nickel concentration is [Ni2+]tot = [Ni2+] + [NiOH+] + [Ni(OH)2] + [Ni(OH].
III As [OH-] increases,the solubility of Ni(OH)2 initially decreases but then increases.
IV
![<strong>Nickel forms three complexes with hydroxide,NiOH<sup>+</sup> (aq),Ni(OH)<sub>2</sub> (aq)and Ni(OH (aq).Which statement(s)regarding the impact of complex formation on the solubility of nickel hydroxide are FALSE? I Hydroxide behaves as a Lewis acid when forming a complex with nickel. II The total nickel concentration is [Ni<sup>2+</sup>]<sub>tot</sub> = [Ni<sup>2+</sup>] + [NiOH<sup>+</sup>] + [Ni(OH)<sub>2</sub>] + [Ni(OH]. III As [OH<sup>-</sup>] increases,the solubility of Ni(OH)<sub>2</sub> initially decreases but then increases. IV </strong> A)Only I B)II and III C)Only III D)Only IV E)I,II,III,and IV](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a3_fc33_a4b1_d3ab1238e74f_TB4000_11.jpg)
A)Only I
B)II and III
C)Only III
D)Only IV
E)I,II,III,and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
5
The molar solubility of a saturated iron(II)carbonate solution derived from the Ksp value is 5.6 x 10−6 M.The molar solubility is greater than 5.6 x 10−6 M when accounting for additional reactions.Which of the reactions below will not increase the solubility of iron(II)carbonate?
A)Fe2+ (aq)+ OH− (aq) FeOH+ (aq)
B)CO32− (aq)+ H+ (aq) HCO3− (aq)
C)HCO3− (aq)+ H+ (aq) H2 CO3 (aq)
D)H2CO3 (aq) CO2 (g)+ H2O (l)
E)FeOH+ (aq)+ OH− Fe(OH)2 (s)
A)Fe2+ (aq)+ OH− (aq) FeOH+ (aq)
B)CO32− (aq)+ H+ (aq) HCO3− (aq)
C)HCO3− (aq)+ H+ (aq) H2 CO3 (aq)
D)H2CO3 (aq) CO2 (g)+ H2O (l)
E)FeOH+ (aq)+ OH− Fe(OH)2 (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
6
All of the following are strong acids,except:
A)HCl.
B)HNO3.
C)HBr.
D)HClO4.
E)HF.
A)HCl.
B)HNO3.
C)HBr.
D)HClO4.
E)HF.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
7
A student attempts to separate Cl− and I− from a solution that is 0.05 M Cl− and 0.10 M I− with Pb(NO3)2.As Pb(NO3)2 is added,which anion will precipitate first and what will the concentration of the anion be when the second anion begins to precipitate? Ksp = 1.7 x 10−5 for PbCl2 and Ksp = 9.8 x 10−9 for PbI2.
A)iodide precipitates first;0.0068 M iodide
B)iodide precipitates first;0.0012 M iodide
C)iodide precipitates first;0.05 M iodide
D)chloride precipitates first;0.0012 chloride
E)chloride precipitates first;0.10 M chloride
A)iodide precipitates first;0.0068 M iodide
B)iodide precipitates first;0.0012 M iodide
C)iodide precipitates first;0.05 M iodide
D)chloride precipitates first;0.0012 chloride
E)chloride precipitates first;0.10 M chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
8
For a reaction with a Ho = +50 kJ and a So = +100.0 J/K, Go is____________________ and the equilibrium constant is____________________ .
A)negative;less than 1
B)negative;greater than 1
C)positive;less than 1
D)positive;greater than 1
E)zero;1
A)negative;less than 1
B)negative;greater than 1
C)positive;less than 1
D)positive;greater than 1
E)zero;1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
9
For the reaction HCN (aq)+ H2O (l) CN- (aq)+ H3O+ (aq),all of the following will shift the equilibrium toward the products,except:
I adding HCN.
II adding H2O.
III removing CN−.
IV removing H3O+.
A)I
B)II
C)I,III,and IV
D)II and IV
E)III
I adding HCN.
II adding H2O.
III removing CN−.
IV removing H3O+.
A)I
B)II
C)I,III,and IV
D)II and IV
E)III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
10
The pH of a solution is 9.65.The solution is________________________________________and the hydroxide concentration is____________________ than the hydronium concentration.
A)acidic;less
B)acidic;greater
C)neutral;equal
D)basic;________________________________________greater
E)basic;less
A)acidic;less
B)acidic;greater
C)neutral;equal
D)basic;________________________________________greater
E)basic;less

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following is INCORRECT for Brønsted-Lowry acids and bases?
A)Acids are proton donors.
B)NH4+ is the conjugate acid of the weak base NH3.
C)When an acid and a base react,the acid and base neutralize each other and form a salt.
D)Water reacts with itself to form hydronium and hydroxide,a process called autoprotolysis.
E)CH3CN is a protic solvent.
A)Acids are proton donors.
B)NH4+ is the conjugate acid of the weak base NH3.
C)When an acid and a base react,the acid and base neutralize each other and form a salt.
D)Water reacts with itself to form hydronium and hydroxide,a process called autoprotolysis.
E)CH3CN is a protic solvent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the pH for a 5 x 10−5 M OH− solution?
A)5)70
B)4)30
C)9)70
D)5)00
E)9)00
A)5)70
B)4)30
C)9)70
D)5)00
E)9)00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which statements below are TRUE for enthalpy and entropy?
I Enthalpy is the heat flow,q,under constant applied pressure.
II Standard conditions for entropy and enthalpy changes are 1 M concentration,1 bar pressure and 298.15 K.
III The entropy change for a system is the amount of energy,at a given temperature,that is dispersed into motion of the molecules in the system.
IV When the value of H is negative,heat is released.When the value of S is negative,entropy is increased.
A)I,II and III
B)I,III and IV
C)II and IV
D)III and IV
E)Another combination is true.
I Enthalpy is the heat flow,q,under constant applied pressure.
II Standard conditions for entropy and enthalpy changes are 1 M concentration,1 bar pressure and 298.15 K.
III The entropy change for a system is the amount of energy,at a given temperature,that is dispersed into motion of the molecules in the system.
IV When the value of H is negative,heat is released.When the value of S is negative,entropy is increased.
A)I,II and III
B)I,III and IV
C)II and IV
D)III and IV
E)Another combination is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
14
Calculate the total nickel concentration for a solution prepared by dissolving Ni(OH)2 (s)in a 0.10 M OH− solution.Ksp = 6 × 10−16, 1 = 1.26 × 104, 2 = 1 × 109,and 3 = 1 × 1012.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
15
Calculate the molar solubility for calcium fluoride in a 0.10 M NaF solution.Ksp = 3.2 × 10−11 for CaF2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
16
Silver ion is being considered as a reagent for separating IO3− from CO32− in a solution that is 0.060 M in K2CO3 and 0.070 M in NaIO3.Which anion will precipitate first and what will its concentration be when the second anion begins to precipitate? Can the two ions be separated with 99.99% efficiency?
Ksp = 3.1 x 10−8 for AgIO3
Ksp = 8.1 x 10−12 for Ag2CO3
Ksp = 3.1 x 10−8 for AgIO3
Ksp = 8.1 x 10−12 for Ag2CO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the classes of acids and bases and strength have been incorrectly matched?
A)RCO2H: weak acid
B)R3N: weak base
C)RCO2−: weak base
D)R3NH+: weak acid
E)R4NOH: weak base
A)RCO2H: weak acid
B)R3N: weak base
C)RCO2−: weak base
D)R3NH+: weak acid
E)R4NOH: weak base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
18
For a triprotic acid,which of the following is NOT true?
A)Ka1Kb3=Kw
B)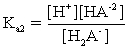
C)Ka1Kb2=Kw
D)Ka3Kb1=Kw
E)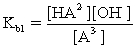
A)Ka1Kb3=Kw
B)
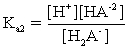
C)Ka1Kb2=Kw
D)Ka3Kb1=Kw
E)
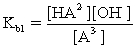

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
19
Calculate the pH for a 0.293 M OH− solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
20
A reaction has a = −167.5 kJ/mol and = 57.3 J/mol K.Calculate the equilibrium constant for the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck








