Deck 13: Edta Titrations
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/20
العب
ملء الشاشة (f)
Deck 13: Edta Titrations
1
The reaction between Al3+ and EDTA is too slow for direct titration.To a 25.00 mL Al3+ sample 50.00 mL of 0.2000 M EDTA is added and allowed to react for 30 minutes.The excess EDTA was titrated with 21.23 mL of 0.1000 M Zn2+.What is the concentration of Al3+ in the sample?
0.3151 M Al3+
2
Calculate [Ba2+] when 35.00 mL of 0.2000 M EDTA is added to 50.00 mL of 0.1000 M Ba2+ in the presence of 0.1 M nitrilotriacetate. ![Calculate [Ba<sup>2+</sup>] when 35.00 mL of 0.2000 M EDTA is added to 50.00 mL of 0.1000 M Ba<sup>2+</sup> in the presence of 0.1 M nitrilotriacetate. = 1.48 x 10<sup>−</sup><sup>4</sup>. = 0.30 for pH 10.K<sub>f</sub> = 7.59 × 10<sup>7</sup> for BaY<sup>2</sup><sup>−</sup>.](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_5387_a4b1_f7b3e4563595_TB4000_11.jpg) = 1.48 x 10−4.
= 1.48 x 10−4. ![Calculate [Ba<sup>2+</sup>] when 35.00 mL of 0.2000 M EDTA is added to 50.00 mL of 0.1000 M Ba<sup>2+</sup> in the presence of 0.1 M nitrilotriacetate. = 1.48 x 10<sup>−</sup><sup>4</sup>. = 0.30 for pH 10.K<sub>f</sub> = 7.59 × 10<sup>7</sup> for BaY<sup>2</sup><sup>−</sup>.](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_5388_a4b1_73c726b0e231_TB4000_11.jpg) = 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.
= 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.
![Calculate [Ba<sup>2+</sup>] when 35.00 mL of 0.2000 M EDTA is added to 50.00 mL of 0.1000 M Ba<sup>2+</sup> in the presence of 0.1 M nitrilotriacetate. = 1.48 x 10<sup>−</sup><sup>4</sup>. = 0.30 for pH 10.K<sub>f</sub> = 7.59 × 10<sup>7</sup> for BaY<sup>2</sup><sup>−</sup>.](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_5387_a4b1_f7b3e4563595_TB4000_11.jpg) = 1.48 x 10−4.
= 1.48 x 10−4. ![Calculate [Ba<sup>2+</sup>] when 35.00 mL of 0.2000 M EDTA is added to 50.00 mL of 0.1000 M Ba<sup>2+</sup> in the presence of 0.1 M nitrilotriacetate. = 1.48 x 10<sup>−</sup><sup>4</sup>. = 0.30 for pH 10.K<sub>f</sub> = 7.59 × 10<sup>7</sup> for BaY<sup>2</sup><sup>−</sup>.](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_5388_a4b1_73c726b0e231_TB4000_11.jpg) = 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.
= 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.7.424 × 10−4 M Mn2+
3
Calculate [Mn2+] when 50.00 mL of 0.1000 M Mn2+ is titrated with 17.00 mL of 0.2000 M EDTA.The titration is buffered to pH 11 for which ![Calculate [Mn<sup>2+</sup>] when 50.00 mL of 0.1000 M Mn<sup>2+</sup> is titrated with 17.00 mL of 0.2000 M EDTA.The titration is buffered to pH 11 for which = 0.81.K<sub>f</sub> = 7.76 × 10<sup>13</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_2c75_a4b1_2bcbe5dcb619_TB4000_11.jpg) = 0.81.Kf = 7.76 × 1013
= 0.81.Kf = 7.76 × 1013
![Calculate [Mn<sup>2+</sup>] when 50.00 mL of 0.1000 M Mn<sup>2+</sup> is titrated with 17.00 mL of 0.2000 M EDTA.The titration is buffered to pH 11 for which = 0.81.K<sub>f</sub> = 7.76 × 10<sup>13</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_2c75_a4b1_2bcbe5dcb619_TB4000_11.jpg) = 0.81.Kf = 7.76 × 1013
= 0.81.Kf = 7.76 × 10130.02388 M Mn2+
4
Which statement is NOT true for auxiliary complexing agents?
A)Auxiliary complexing agents prevent the precipitation of metal hydroxide at high pH.
B)Auxiliary complexing agents are chosen to bind metal strong enough to prevent metal hydroxide precipitation but weak enough to be displaced by EDTA.
C)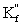
Is the effective formation constant for fixed pH and fixed concentration auxiliary complexing agent.
D)Low concentrations auxiliary complexing agent obliterates the end point of the titration.
E)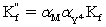
A)Auxiliary complexing agents prevent the precipitation of metal hydroxide at high pH.
B)Auxiliary complexing agents are chosen to bind metal strong enough to prevent metal hydroxide precipitation but weak enough to be displaced by EDTA.
C)
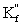
Is the effective formation constant for fixed pH and fixed concentration auxiliary complexing agent.
D)Low concentrations auxiliary complexing agent obliterates the end point of the titration.
E)
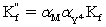

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
5
Calculate pBa when 35.00 mL of 0.1 M EDTA is added to 50.00 mL of 0.1 M Ba2+ in the presence of 0.1 M nitrilotriacetate. 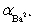 = 1.48 × 10−4.
= 1.48 × 10−4. 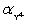 = 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.
= 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.
A)4)82
B)2)64
C)1)75
D)4)55
E)3)53
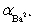 = 1.48 × 10−4.
= 1.48 × 10−4. 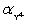 = 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.
= 0.30 for pH 10.Kf = 7.59 × 107 for BaY2−.A)4)82
B)2)64
C)1)75
D)4)55
E)3)53

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
6
A____________________ is a reagent that protects a component of the analyte from reaction with EDTA.
A)hindrance agent
B)displacement agent
C)masking agent
D)blocking agent
E)reducing agent
A)hindrance agent
B)displacement agent
C)masking agent
D)blocking agent
E)reducing agent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
7
Ammonia,0.10 M,is used as an auxiliary complexing agent for a Co2+ titration with EDTA.There are 6 s for the complex between ammonia and Co2+.Which equation is used to calculate 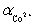 ?
?
A)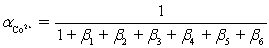
B)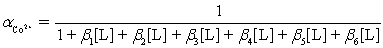
C)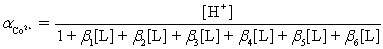
D)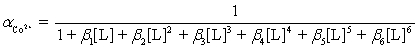
E)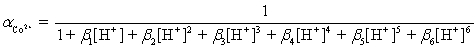
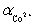 ?
?A)
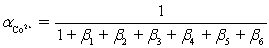
B)
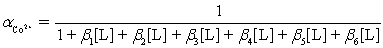
C)
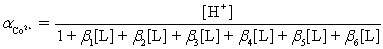
D)
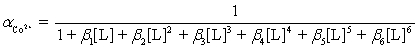
E)
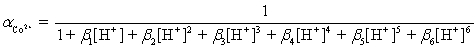

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
8
The____________________ is the ability of multidentate ligands to form more stable metal complexes than those formed by similar monodentate ligands.
A)ligand effect
B)multidentate effect
C)chelate effect
D)Lewis effect
E)isomer effect
A)ligand effect
B)multidentate effect
C)chelate effect
D)Lewis effect
E)isomer effect

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which is NOT an end point detection method used with EDTA titrations?
A)metal ion indicators
B)adsorption indicators
C)glass (pH)electrode
D)mercury electrode
E)ion-selective electrode
A)metal ion indicators
B)adsorption indicators
C)glass (pH)electrode
D)mercury electrode
E)ion-selective electrode

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
10
Calculate pBa when 35.00 mL 0.1 M EDTA is added to 50.00 mL of 0.1 M Ba2+.For the buffered pH of 10, 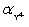 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.
A)1)39
B)1)75
C)4)56
D)4)82
E)4)70
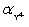 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.A)1)39
B)1)75
C)4)56
D)4)82
E)4)70

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
11
For EDTA titrations the titration reaction is Mn+ + EDTA ⇋ MYn-4.Which statements are true for EDTA titration curves?
I At the equivalence point,there is exactly as much EDTA in solution as metal.[Mn+] is calculated from the dissociation of MYn-4 formed.
II After the equivalence point,the concentration free EDTA equals excess EDTA and virtually all metal ion is in the form MYn-4.[Mn+] is calculated from the dissociation of MYn-4.
III Before the equivalence point,free [Mn+] equals excess unreacted Mn+ after EDTA has been consumed.Dissociation of MYn-4 is negligible.
IV pM is plotted on the y-axis and milliliters EDTA solution added on the x-axis.
A)I,II,and III
B)I,III,and IV
C)III and IV
D)I,II,III,and IV
E)II,III,and IV
I At the equivalence point,there is exactly as much EDTA in solution as metal.[Mn+] is calculated from the dissociation of MYn-4 formed.
II After the equivalence point,the concentration free EDTA equals excess EDTA and virtually all metal ion is in the form MYn-4.[Mn+] is calculated from the dissociation of MYn-4.
III Before the equivalence point,free [Mn+] equals excess unreacted Mn+ after EDTA has been consumed.Dissociation of MYn-4 is negligible.
IV pM is plotted on the y-axis and milliliters EDTA solution added on the x-axis.
A)I,II,and III
B)I,III,and IV
C)III and IV
D)I,II,III,and IV
E)II,III,and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
12
Calculate [Mn2+] when 50.00 mL of 0.1000 M Mn2+ is titrated with 25.00 mL of 0.2000 M EDTA.The titration is buffered to pH 11 for which ![Calculate [Mn<sup>2+</sup>] when 50.00 mL of 0.1000 M Mn<sup>2+</sup> is titrated with 25.00 mL of 0.2000 M EDTA.The titration is buffered to pH 11 for which = 0.81.K<sub>f</sub> = 7.76 × 10<sup>13</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_5386_a4b1_a71a70d811b4_TB4000_11.jpg) = 0.81.Kf = 7.76 × 1013
= 0.81.Kf = 7.76 × 1013
![Calculate [Mn<sup>2+</sup>] when 50.00 mL of 0.1000 M Mn<sup>2+</sup> is titrated with 25.00 mL of 0.2000 M EDTA.The titration is buffered to pH 11 for which = 0.81.K<sub>f</sub> = 7.76 × 10<sup>13</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25a9_5386_a4b1_a71a70d811b4_TB4000_11.jpg) = 0.81.Kf = 7.76 × 1013
= 0.81.Kf = 7.76 × 1013
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which statement is NOT true?
A)A monodentate ligand binds to a metal atom through only one atom.
B)A metal is multidentate if it can bind to more than one ligand.
C)Metal ions are Lewis acids.
D)Ligands are Lewis bases.
E)Most transition metals bind six ligand atoms.
A)A monodentate ligand binds to a metal atom through only one atom.
B)A metal is multidentate if it can bind to more than one ligand.
C)Metal ions are Lewis acids.
D)Ligands are Lewis bases.
E)Most transition metals bind six ligand atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
14
EDTA is a hexadentate ligand containing four carboxylic acid groups and two amines.Which is true for the acid-base properties of EDTA?
I EDTA is a hexaprotic system,H6Y2+,when the amines are protonated.
II Neutral EDTA,H4Y,is tetraprotic when the amines are not protonated.
III Deprotonated EDTA,Y4−,is the only form of EDTA that binds to metal cations.
IV The fraction of EDTA in any of the six EDTA species is pH dependent.
A)III and IV
B)I and II
C)I,III,and IV
D)II,III,and IV
E)I,II,and IV
I EDTA is a hexaprotic system,H6Y2+,when the amines are protonated.
II Neutral EDTA,H4Y,is tetraprotic when the amines are not protonated.
III Deprotonated EDTA,Y4−,is the only form of EDTA that binds to metal cations.
IV The fraction of EDTA in any of the six EDTA species is pH dependent.
A)III and IV
B)I and II
C)I,III,and IV
D)II,III,and IV
E)I,II,and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
15
Calculate pBa when 50.00 mL 0.1 M EDTA is added to 50.00 mL of 0.1 M Ba2+.For the buffered pH of 10, 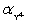 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.
A)1)30
B)7)88
C)4)59
D)7)36
E)4)33
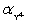 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.A)1)30
B)7)88
C)4)59
D)7)36
E)4)33

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
16
Calculate 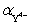 for a 0.2 M EDTA solution buffered at pH 10.00.For EDTA K1 = 1.0,K2 = 31.6,K3 = 100,K4 = 490,K5 = 1.35 × 106 and K6 = 2.34 × 1010.
for a 0.2 M EDTA solution buffered at pH 10.00.For EDTA K1 = 1.0,K2 = 31.6,K3 = 100,K4 = 490,K5 = 1.35 × 106 and K6 = 2.34 × 1010.
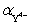 for a 0.2 M EDTA solution buffered at pH 10.00.For EDTA K1 = 1.0,K2 = 31.6,K3 = 100,K4 = 490,K5 = 1.35 × 106 and K6 = 2.34 × 1010.
for a 0.2 M EDTA solution buffered at pH 10.00.For EDTA K1 = 1.0,K2 = 31.6,K3 = 100,K4 = 490,K5 = 1.35 × 106 and K6 = 2.34 × 1010.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
17
A____________________ titration is necessary for analytes that precipitates in the absence of EDTA,that reacts slowly with EDTA or that blocks the indicator.
A)direct
B)displacement
C)indirect
D)masking
E)back
A)direct
B)displacement
C)indirect
D)masking
E)back

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
18
Calculate pBa when 75.00 mL 0.1 M EDTA is added to 50.00 mL of 0.1 M Ba2+.For the buffered pH of 10, 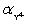 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.
A)7)06
B)3)53
C)4)33
D)7)58
E)4)59
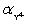 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.A)7)06
B)3)53
C)4)33
D)7)58
E)4)59

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which statement is incorrect for conditional formation constants,Kf′?
A)Kf′ is pH dependent.
B)Kf′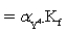
C)Kf′ allows the view of uncomplexed EDTA as being in only one form,Y4−,in equilibrium calculations.
D)As pH drops the value of Kf′ increases.
E)The values of Kf′ must be large to guarantee sharp end points during titrations.
A)Kf′ is pH dependent.
B)Kf′
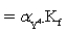
C)Kf′ allows the view of uncomplexed EDTA as being in only one form,Y4−,in equilibrium calculations.
D)As pH drops the value of Kf′ increases.
E)The values of Kf′ must be large to guarantee sharp end points during titrations.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
20
A metal that does not freely dissociate from an indicator is said to____________________ the indicator.
A)hinder
B)block
C)restrict
D)impair
E)handicap
A)hinder
B)block
C)restrict
D)impair
E)handicap

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck








