Deck 15: Fundamentals of Electrochemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/20
العب
ملء الشاشة (f)
Deck 15: Fundamentals of Electrochemistry
1
For the line notation below,which is NOT true? Fe(s)| Fe2+ (aq,A = 1)|| Cu2+ (aq,A = 1)| Cu (s)
A)Fe is the anode.
B)|| represents the salt bridge.
C)Cu is the anode.
D)1 M Cu2+ is the electrolyte concentration and species for the copper electrode.
E)1 M Fe2+ is the electrolyte concentration and species for the iron electrode.
A)Fe is the anode.
B)|| represents the salt bridge.
C)Cu is the anode.
D)1 M Cu2+ is the electrolyte concentration and species for the copper electrode.
E)1 M Fe2+ is the electrolyte concentration and species for the iron electrode.
Cu is the anode.
2
Calculate the equilibrium constant for the reaction between Sn metal and Zn2+ solution. Sn + Zn2+ ⇋ Sn2+ + Zn
Sn2+ + 2 e− → Sn (s)E° = −0.141 V
Zn2+ + 2 e− → Zn (s)E° = −0.762 V
A)9)86 × 1020
B)1)21 × 10−21
C)3)37 × 1030
D)3)18 × 10−11
E)1)84 × 1015
Sn2+ + 2 e− → Sn (s)E° = −0.141 V
Zn2+ + 2 e− → Zn (s)E° = −0.762 V
A)9)86 × 1020
B)1)21 × 10−21
C)3)37 × 1030
D)3)18 × 10−11
E)1)84 × 1015
1)21 × 10−21
3
Calculate E°' for the half-cell below. O2 + 4 H+ + 4 e− ⇋ 2 H2O E° = 1.229 V
A)1.643 V
B)0.815 V
C)0.307 V
D)1.229 V
E)0.711 V
A)1.643 V
B)0.815 V
C)0.307 V
D)1.229 V
E)0.711 V
0.815 V
4
For the silver half-reaction,Ag+ + 1 e- → Ag (s),when the concentration of silver cation is increased,the reduction potential:
A)becomes more positive.
B)becomes more negative.
C)remains constant.
D)increases or decreases depending on the voltage of the other half-reaction.
E)increases or decreases depending on the temperature.
A)becomes more positive.
B)becomes more negative.
C)remains constant.
D)increases or decreases depending on the voltage of the other half-reaction.
E)increases or decreases depending on the temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
5
Calculate Eo and K for the cell composed of a bromate/bromide half-cell and a chlorate/chlorine dioxide half-cell. 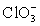 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 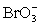 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
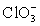 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 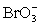 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
6
For the cell diagram below write out and balance the reaction that occurs in the cell.
Pt|HOCl (aq,1 M),HClO2 (aq,1 M),H+ (aq,1 M)||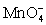 (aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
(aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
Pt|HOCl (aq,1 M),HClO2 (aq,1 M),H+ (aq,1 M)||
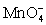 (aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
(aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
7
All of the following are true for the measurement of standard reduction potentials,except:
I standard conditions for reduction potentials is 25 oC and A = 1 for all aqueous and gaseous species.
II reduction potentials are measured relative to the standard hydrogen electrode (S.H.E. ).
III standard reduction potentials are measured while connected to the negative electrode of the potentiometer.
IV the E° for the S.H.E.is defined as 0 volts.
A)I and IV
B)I and III
C)I only
D)III only
E)II and IV
I standard conditions for reduction potentials is 25 oC and A = 1 for all aqueous and gaseous species.
II reduction potentials are measured relative to the standard hydrogen electrode (S.H.E. ).
III standard reduction potentials are measured while connected to the negative electrode of the potentiometer.
IV the E° for the S.H.E.is defined as 0 volts.
A)I and IV
B)I and III
C)I only
D)III only
E)II and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
8
Calculate the standard potential when the two half-cells below are connected via a salt bridge and potentiometer.Which species are the reducing agent and oxidizing agent? 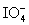 + 2 H+ + 2 e- ⇋
+ 2 H+ + 2 e- ⇋ 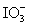 + H2O E° = 1.589 V
+ H2O E° = 1.589 V
Mg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V
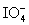 + 2 H+ + 2 e- ⇋
+ 2 H+ + 2 e- ⇋ 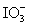 + H2O E° = 1.589 V
+ H2O E° = 1.589 VMg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
9
A bromide probe is constructed from a saturated silver bromide half-cell and a Ni2+/Ni half-cell.If the measured potential is 0.952 V and [Ni2+] = 1 M,what is the bromide concentration?
AgBr (s)+ e− ⇋ Ag(s)+ Br− E° = 0.071 V
Ni2+ + 2 e− ⇋ Ni (s)E° = −0.714 V
AgBr (s)+ e− ⇋ Ag(s)+ Br− E° = 0.071 V
Ni2+ + 2 e− ⇋ Ni (s)E° = −0.714 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
10
For the reaction below,the oxidizing agent is____________________ and it____________________ electrons,and the reducing agent is____________________ and it____________________ electrons. 8 H+ + 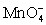 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
A)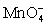
;gains 5;Cu+;loses 1
B)H+;loses 1;Cu+;gains 1
C)H+;gains 1;
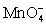
;loses 5
D)Cu+;loses 1;
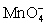
;gains 1
E)Cu+;loses 1;H+;gains 1
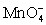 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2OA)
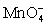
;gains 5;Cu+;loses 1
B)H+;loses 1;Cu+;gains 1
C)H+;gains 1;
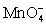
;loses 5
D)Cu+;loses 1;
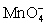
;gains 1
E)Cu+;loses 1;H+;gains 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
11
A galvanic cell is at equilibrium when:
A)Ecell is a constant value.
B)cell resistance is very low.
C)Ecell = 0 V.
D)Ecell fluctuates between E°cell.
E)cell resistance is infinitely large.
A)Ecell is a constant value.
B)cell resistance is very low.
C)Ecell = 0 V.
D)Ecell fluctuates between E°cell.
E)cell resistance is infinitely large.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which is true when electrochemical cells are used as chemical probes?
I.All concentrations in the half-cells must be known except for the analyte of interest.
II. The analyte concentration is calculated from the equilibrium cell potential.
III. Equilibrium between the half-cells is established.
IV. Equilibrium within each half-cell is established and is assumed to remain at equilibrium.
V. Half-cells other than the S.H.E.can be used to determine pH.
A)II,III,and IV
B)I,IV,and V
C)I and II
D)III,IV,and V
E)I,II,and V
I.All concentrations in the half-cells must be known except for the analyte of interest.
II. The analyte concentration is calculated from the equilibrium cell potential.
III. Equilibrium between the half-cells is established.
IV. Equilibrium within each half-cell is established and is assumed to remain at equilibrium.
V. Half-cells other than the S.H.E.can be used to determine pH.
A)II,III,and IV
B)I,IV,and V
C)I and II
D)III,IV,and V
E)I,II,and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which is NOT correct for when the silver and vanadium half-cells are connected via a salt bridge and a potentiometer?
Ag+ + 1 e- → Ag (s)E° = 0.7993 V
V2+ + 2 e- → V (s)E° = −1.125 V
I Ag+ is reduced.
II V is oxidized.
III Eocell = 1.924 V
IV V2+ is reduced.
V Ag is oxidized.
A)I and II
B)III,IV,and V
C)I,II,and III
D)III only
E)IV and V
Ag+ + 1 e- → Ag (s)E° = 0.7993 V
V2+ + 2 e- → V (s)E° = −1.125 V
I Ag+ is reduced.
II V is oxidized.
III Eocell = 1.924 V
IV V2+ is reduced.
V Ag is oxidized.
A)I and II
B)III,IV,and V
C)I,II,and III
D)III only
E)IV and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
14
Eo' is defined as:
A)the formal half-cell potential for compounds that are neither acids nor bases.
B)the formal half-cell potential at 20 C.
C)the formal half-cell potential at pH 7.
D)the inverse of E°.
E)None of these answers is the definition of E°'.
A)the formal half-cell potential for compounds that are neither acids nor bases.
B)the formal half-cell potential at 20 C.
C)the formal half-cell potential at pH 7.
D)the inverse of E°.
E)None of these answers is the definition of E°'.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
15
The half-cells below are connected via a salt-bridge and potentiometer.What is the voltage read by the potentiometer when AAg+ = 2.00 and AV2+ = 1.50? Ag+ + 1 e− → Ag (s)Eo = 0.7993 V
V2+ + 2 e− → V (s)Eo = −1.125 V
A)1)912 V
B)−0.326 V
C)−0.313 V
D)1)937 V
E)1)924 V
V2+ + 2 e− → V (s)Eo = −1.125 V
A)1)912 V
B)−0.326 V
C)−0.313 V
D)1)937 V
E)1)924 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following is NOT true for galvanic cells?
A)Galvanic cells are spontaneous.
B)Oxidation occurs at the anode and reduction occurs at the cathode.
C)Electrons move toward more negative electrical potential.
D)Galvanic cells are composed of two half-cells connected by salt bridge.
E)The salt bridge maintains electroneutrality throughout the cell.
A)Galvanic cells are spontaneous.
B)Oxidation occurs at the anode and reduction occurs at the cathode.
C)Electrons move toward more negative electrical potential.
D)Galvanic cells are composed of two half-cells connected by salt bridge.
E)The salt bridge maintains electroneutrality throughout the cell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
17
A cell is constructed according to the cell diagram below.If the measured potential is 1.00 V,what is the pH of the dichromate cell? Fe|Fe2+ (aq,1M)|| 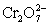 (aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt
(aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt 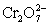 + 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
+ 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
Fe2+ + 2 e− ⇋ Fe (s)____________________ E° = −0.44 V
A)1)931
B)5)797
C)12.162
D)6)081
E)4)054
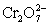 (aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt
(aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt 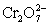 + 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
+ 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 VFe2+ + 2 e− ⇋ Fe (s)____________________ E° = −0.44 V
A)1)931
B)5)797
C)12.162
D)6)081
E)4)054

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which relationship is NOT correctly defined?
A)Work = E. q
B)( G = -n · F · E)
C)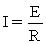
D)P = E . I
E)q = n .N .F
A)Work = E. q
B)( G = -n · F · E)
C)
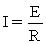
D)P = E . I
E)q = n .N .F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
19
Calculate the potential for the half-cell below for [ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7b_a4b1_3547427d4325_TB4000_11.jpg) ] = 0.100 M,[
] = 0.100 M,[ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7c_a4b1_11f3bec3a0eb_TB4000_11.jpg) ] = 0.500 M and the pH = 10.50.
] = 0.500 M and the pH = 10.50.
2![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7d_a4b1_e907d93947e4_TB4000_11.jpg) + 3 H2O + 4 e− ⇋
+ 3 H2O + 4 e− ⇋ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7e_a4b1_710927175ef4_TB4000_11.jpg) + 6 OH- E° = −0.566 V
+ 6 OH- E° = −0.566 V
![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7b_a4b1_3547427d4325_TB4000_11.jpg) ] = 0.100 M,[
] = 0.100 M,[ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7c_a4b1_11f3bec3a0eb_TB4000_11.jpg) ] = 0.500 M and the pH = 10.50.
] = 0.500 M and the pH = 10.50.2
![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7d_a4b1_e907d93947e4_TB4000_11.jpg) + 3 H2O + 4 e− ⇋
+ 3 H2O + 4 e− ⇋ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://d2lvgg3v3hfg70.cloudfront.net/TB4000/11ea6952_25ad_4b7e_a4b1_710927175ef4_TB4000_11.jpg) + 6 OH- E° = −0.566 V
+ 6 OH- E° = −0.566 V
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck
20
A galvanic cell with a large equilibrium constant has a____________________ cell potential.
A)negative
B)constant
C)zero voltage
D)positive
E)variable
A)negative
B)constant
C)zero voltage
D)positive
E)variable

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 20 في هذه المجموعة.
فتح الحزمة
k this deck








