Deck 5: Water for Life
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/74
العب
ملء الشاشة (f)
Deck 5: Water for Life
1
Which is the best definition of specific heat?
A)the quantity of heat energy that must be absorbed to cause one gram of a liquid substance to boil
B)the quantity of heat energy that must be absorbed to increase the temperature of one gram of a substance one degree Celsius
C)the boiling point of a liquid substance in degrees Celsius
D)the difference between the freezing point and the boiling point of a substance
A)the quantity of heat energy that must be absorbed to cause one gram of a liquid substance to boil
B)the quantity of heat energy that must be absorbed to increase the temperature of one gram of a substance one degree Celsius
C)the boiling point of a liquid substance in degrees Celsius
D)the difference between the freezing point and the boiling point of a substance
the quantity of heat energy that must be absorbed to increase the temperature of one gram of a substance one degree Celsius
2
How many moles of sodium chloride are there in 250 mL of a 1.20 M sodium chloride solution?
A)0.30
B)0.60
C)1.2
D)2.4
A)0.30
B)0.60
C)1.2
D)2.4
0.30
3
What percentage of Earth's water is in the oceans?
A)97.4%
B)50%
C)2.59%
D)0.014%
A)97.4%
B)50%
C)2.59%
D)0.014%
97.4%
4
Numbers 1 through 4 are used to identify four different elements.Based on periodic table trends,which number identifies the element that is expected to have the greatest tendency to attract a shared pair of electrons? 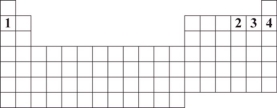
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
5
If the lead concentration in water is 1 ppm,then we should be able to recover 1 mg of lead from ____ L of water.
A)0.01
B)0.1
C)1
D)10
A)0.01
B)0.1
C)1
D)10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
6
The recommended daily requirement of calcium is 1,000 mg.Tap water in a Midwestern city contains approximately 150 mg Ca/L.A person living in the city who drinks two liters of water in one day would receive ________ percent of his/her RDA of calcium.
A)3
B)7.5
C)15
D)30
A)3
B)7.5
C)15
D)30

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
7
"Hard" water in parts of the Midwest may have a calcium ion concentration as high as 400 ppm.What is this calcium ion concentration when expressed as a percentage?
A)40%
B)4%
C)0.4%
D)0.04%
A)40%
B)4%
C)0.4%
D)0.04%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
8
Electronegativity
A)is a relative measure of an atom's attraction for electrons it shares in a covalent bond.
B)is the total negative charge on a polyatomic anion.
C)is the same for all of the elements in a family or group.
D)decreases from left to right across a period on the periodic table.
A)is a relative measure of an atom's attraction for electrons it shares in a covalent bond.
B)is the total negative charge on a polyatomic anion.
C)is the same for all of the elements in a family or group.
D)decreases from left to right across a period on the periodic table.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
9
The fact that carbon (C)is less electronegative than nitrogen (N)means that in a C N bond,the
A)shared electrons are closer to the C atom than to the N atom.
B)shared electrons are closer to the N atom than to the C atom.
C)C atom takes the electrons from the N atom forming C¯ and N+.
D)N atom takes the electrons from the C atom forming C+ and N¯.
A)shared electrons are closer to the C atom than to the N atom.
B)shared electrons are closer to the N atom than to the C atom.
C)C atom takes the electrons from the N atom forming C¯ and N+.
D)N atom takes the electrons from the C atom forming C+ and N¯.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
10
A student wants to prepare exactly 250 mL of a 0.500 M aqueous potassium hydroxide solution.What mass of potassium hydroxide (molar mass = 56.10 g/mol)must the student dissolve in the 250 mL of solution?
A)56.1 g
B)28.1 g
C)14.0 g
D)7.01 g
A)56.1 g
B)28.1 g
C)14.0 g
D)7.01 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is an aquifer?
A)a holding tank for disinfected water at the municipal water works
B)a water purification system developed by the Romans
C)a large pool of water trapped in sand and gravel below the surface of the earth
D)a solution in which the solvent is water
A)a holding tank for disinfected water at the municipal water works
B)a water purification system developed by the Romans
C)a large pool of water trapped in sand and gravel below the surface of the earth
D)a solution in which the solvent is water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
12
A polar covalent bond is created when
A)a metallic element reacts with a nonmetallic element.
B)two atoms share their bonding electrons unequally.
C)two atoms share three or more electrons.
D)two atoms of the same element form double or triple bonds.
A)a metallic element reacts with a nonmetallic element.
B)two atoms share their bonding electrons unequally.
C)two atoms share three or more electrons.
D)two atoms of the same element form double or triple bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
13
The drawing shows two water molecules.Which statement is correct? 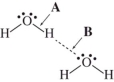
A)A: covalent bond;B: hydrogen bond;B is stronger
B)A: hydrogen bond;B: covalent bond;A is stronger
C)A: covalent bond;B: hydrogen bond;A is stronger
D)A: hydrogen bond;B: covalent bond;B is stronger

A)A: covalent bond;B: hydrogen bond;B is stronger
B)A: hydrogen bond;B: covalent bond;A is stronger
C)A: covalent bond;B: hydrogen bond;A is stronger
D)A: hydrogen bond;B: covalent bond;B is stronger

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which molecule(s)contain(s)polar covalent bonds,but is(are)nonpolar? 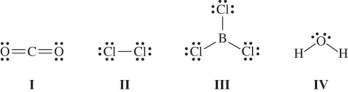
A)I only
B)III only
C)II and IV only
D)I and III only

A)I only
B)III only
C)II and IV only
D)I and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the molarity of sodium chloride in a solution containing 0.50 mol of sodium chloride in 500 mL of water?
A)0.25 M
B)0.50 M
C)1.0 M
D)5.0 M
A)0.25 M
B)0.50 M
C)1.0 M
D)5.0 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
16
Generally,the water that has the least harmful contaminants is
A)water from the oceans.
B)surface water from lakes.
C)groundwater from aquifers.
D)surface water from rivers.
A)water from the oceans.
B)surface water from lakes.
C)groundwater from aquifers.
D)surface water from rivers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which correctly describes groundwater?
A)any water found above ground in lakes and rivers;usually needs treatment to remove harmful contaminants
B)any water found above ground in lakes and rivers;usually free of harmful contaminants
C)any water taken from aquifers
D)none of the above.
A)any water found above ground in lakes and rivers;usually needs treatment to remove harmful contaminants
B)any water found above ground in lakes and rivers;usually free of harmful contaminants
C)any water taken from aquifers
D)none of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
18
A 4-L sample of water contains 80 µg of lead.What is this lead concentration,in parts per billion (ppb)?
A)20
B)80
C)320
D)500
A)20
B)80
C)320
D)500

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
19
In general,hydrogen bonds are
A)about ten times stronger than typical covalent bonds.
B)about 1/10 as strong as typical covalent bonds.
C)found in every molecule containing more than one hydrogen atom.
D)found only between H atoms in molecules like H2.
A)about ten times stronger than typical covalent bonds.
B)about 1/10 as strong as typical covalent bonds.
C)found in every molecule containing more than one hydrogen atom.
D)found only between H atoms in molecules like H2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
20
In general,hydrogen bonds are ____ and _____ typical covalent bonds.
A)longer than;about one tenth as strong as
B)shorter than;about one tenth as strong as
C)longer than;about ten times as strong as
D)shorter than;about ten times as strong as
A)longer than;about one tenth as strong as
B)shorter than;about one tenth as strong as
C)longer than;about ten times as strong as
D)shorter than;about ten times as strong as

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
21
A 0.25 M solution of the sugar sucrose,C12H22O11,in water is tested for conductivity using the type of apparatus shown.What do you predict will happen? 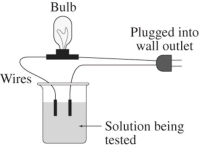
A)The bulb will not light up.Sucrose is an electrolyte,but not very soluble in aqueous solution.
B)The bulb will not light up.Sucrose is in the molecular form in aqueous solution.
C)The light bulb will shine dimly.Sucrose is only partially ionized in aqueous solution.
D)The light bulb will shine brightly.Sucrose is highly ionized in aqueous solution.

A)The bulb will not light up.Sucrose is an electrolyte,but not very soluble in aqueous solution.
B)The bulb will not light up.Sucrose is in the molecular form in aqueous solution.
C)The light bulb will shine dimly.Sucrose is only partially ionized in aqueous solution.
D)The light bulb will shine brightly.Sucrose is highly ionized in aqueous solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which best symbolizes the hydrogen bonding between two water molecules? 
A)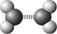
B)
C)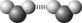
D)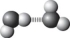

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the correct name for (NH4)2CO3?
A)biammonium carbonate
B)ammonium bicarbonate
C)ammonium carboxide
D)ammonium carbonate
A)biammonium carbonate
B)ammonium bicarbonate
C)ammonium carboxide
D)ammonium carbonate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which shows the Lewis structure of water with the correct partial charges and nonbonding electrons?
A)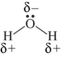
B)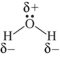
C)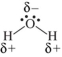
D)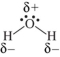
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which statement is not true?
A)In forming a solution,the solute dissolves other substances that are called solvents.
B)Water is the solvent in an aqueous solution.
C)Pure water does not conduct electricity.
D)Electrolytes dissolved in water produce a solution that can conduct electricity.
A)In forming a solution,the solute dissolves other substances that are called solvents.
B)Water is the solvent in an aqueous solution.
C)Pure water does not conduct electricity.
D)Electrolytes dissolved in water produce a solution that can conduct electricity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which is the best representation of the solvation of a sodium cation in water? 
A)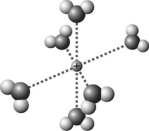
B)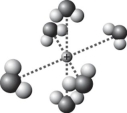
C)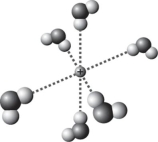
D)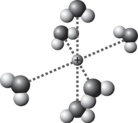
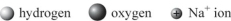
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
27
The formula for a sulfate ion is SO42¯.What is the formula of gallium (Ga)sulfate?
A)GaSO4
B)Ga2SO4
C)Ga2(SO4)3
D)Ga3(SO4)2
A)GaSO4
B)Ga2SO4
C)Ga2(SO4)3
D)Ga3(SO4)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
28
Most polyatomic ions
A)have a negative charge.
B)are composed of a total of two atoms.
C)are cations.
D)contain sulfur atoms.
A)have a negative charge.
B)are composed of a total of two atoms.
C)are cations.
D)contain sulfur atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
29
The correct name for CuO is
A)copper oxide.
B)monocopper oxide.
C)copper(I)oxide.
D)copper(II)oxide.
A)copper oxide.
B)monocopper oxide.
C)copper(I)oxide.
D)copper(II)oxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which is the correct Lewis structure of a chlorine atom and the ion that chlorine forms?
A)
B)
C)
D)
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
31
Water has one of the highest specific heats of any known liquid.This means that the temperature of a water sample _____ with the input of a _____ amount of energy.
A)decreases greatly;small
B)decreases only slightly;large
C)increases only slightly;large
D)increases greatly;small
A)decreases greatly;small
B)decreases only slightly;large
C)increases only slightly;large
D)increases greatly;small

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
32
The numbers 1 through 4 are used to identify four different elements on the periodic table.Which element is expected to have 2+ charge when it forms an ion? 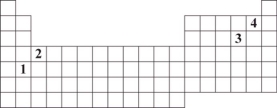
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which is not a consequence of hydrogen bonding between water molecules?
A)Water has a high boiling point.
B)Ice floats on water.
C)Water has a high specific heat.
D)Water is colorless and odorless.
A)Water has a high boiling point.
B)Ice floats on water.
C)Water has a high specific heat.
D)Water is colorless and odorless.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which is the correct formula (including charge)of a nitrate ion?
A)NO2+
B)NO2¯
C)NO3¯
D)NO3+
A)NO2+
B)NO2¯
C)NO3¯
D)NO3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
35
When the ________molecules of ethanol,C2H5OH,are added to water,the ethanol molecules
A)nonpolar;are attracted to the nonpolar water molecules.
B)polar;form hydrogen bonds with the polar water molecules.
C)polar;form covalent bonds with the polar water molecules.
D)nonpolar;are not attracted to the polar water molecules.
A)nonpolar;are attracted to the nonpolar water molecules.
B)polar;form hydrogen bonds with the polar water molecules.
C)polar;form covalent bonds with the polar water molecules.
D)nonpolar;are not attracted to the polar water molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which corresponds to the composition of the ion typically formed by magnesium? 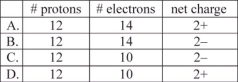
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
37
A 0.25 M aqueous solution of potassium chloride,KCl,is tested for conductivity using the type of apparatus shown.What do you predict will happen? 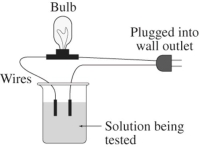
A)The bulb will not light up.KCl is a nonelectrolyte.
B)The bulb will not light up.KCl is in the molecular form when dissolved in water.
C)The light bulb will shine dimly.KCl is only partially ionized in aqueous solution.
D)The light bulb will shine brightly.KCl is highly ionized in aqueous solution.

A)The bulb will not light up.KCl is a nonelectrolyte.
B)The bulb will not light up.KCl is in the molecular form when dissolved in water.
C)The light bulb will shine dimly.KCl is only partially ionized in aqueous solution.
D)The light bulb will shine brightly.KCl is highly ionized in aqueous solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the formula of the ionic compound formed from magnesium,Mg,and chlorine,Cl?
A)MgCl
B)Mg2Cl2
C)Mg2Cl
D)MgCl2
A)MgCl
B)Mg2Cl2
C)Mg2Cl
D)MgCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
39
How many joules are required to heat 2.0 L of water from 20°C to its boiling point of 100°C? The specific heat of water is 4.18 J/g•°C and the density of water is 1 g/mL.
A)6.7 × 105 J
B)1.7 × 105 J
C)3.8 × 104 J
D)6.7 × 102 J
A)6.7 × 105 J
B)1.7 × 105 J
C)3.8 × 104 J
D)6.7 × 102 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which corresponds to the composition of the ion typically formed by fluorine? 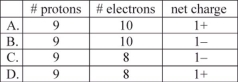
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following is/are true about Maximum Contaminant Level Goals (MCLGs)?
I.The MCLG for any contaminant that is regulated as a carcinogen is set to zero.
II.An average person weighing 70 kg (154 lb)could drink two liters (about two quarts)of water containing a contaminant at the MCGL level every day for 70 years without suffering any ill effects.
III.The Maximum Contaminant Level (MCL)set by the EPA may be higher than the MCLG to account for the financial and technical barriers that may make achieving these goals difficult.
IV.The MCLG for a contaminant is always set to zero because this is the best way to protect human health.
A)II only
B)I and II only
C)I,II,and III only
D)I,II,III,and IV
I.The MCLG for any contaminant that is regulated as a carcinogen is set to zero.
II.An average person weighing 70 kg (154 lb)could drink two liters (about two quarts)of water containing a contaminant at the MCGL level every day for 70 years without suffering any ill effects.
III.The Maximum Contaminant Level (MCL)set by the EPA may be higher than the MCLG to account for the financial and technical barriers that may make achieving these goals difficult.
IV.The MCLG for a contaminant is always set to zero because this is the best way to protect human health.
A)II only
B)I and II only
C)I,II,and III only
D)I,II,III,and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which is not a form of chlorine used to disinfect water?
A)chlorine gas
B)chloroform
C)sodium hypochlorite
D)calcium hypochlorite
A)chlorine gas
B)chloroform
C)sodium hypochlorite
D)calcium hypochlorite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
43
When table sugar (sucrose,C12H22O11)dissolves in water,which type(s)of attraction between water and sugar molecules occur(s)?
I.hydrogen bonding
II.polar-polar interactions
III.covalent bonding
A)I and II only
B)II and III only
C)I and III only
D)I,II,and III
I.hydrogen bonding
II.polar-polar interactions
III.covalent bonding
A)I and II only
B)II and III only
C)I and III only
D)I,II,and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
44
At water treatment plants aluminum sulfate and calcium hydroxide are added to
A)neutralize excess ozone.
B)neutralize excess chlorine.
C)remove suspended clay and dust particles.
D)neutralize excess acidity or basicity.
A)neutralize excess ozone.
B)neutralize excess chlorine.
C)remove suspended clay and dust particles.
D)neutralize excess acidity or basicity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
45
Potable water is water that
A)contains residual chlorine.
B)is fit for human consumption.
C)is contaminated with lead.
D)contains too much residual ozone to be drinkable.
A)contains residual chlorine.
B)is fit for human consumption.
C)is contaminated with lead.
D)contains too much residual ozone to be drinkable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of these is not a trihalomethane?
A)CHCl3
B)CH3Cl
C)CHF3
D)CHBr2Cl
A)CHCl3
B)CH3Cl
C)CHF3
D)CHBr2Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
47
The EPA regulates the presence of nitrates and nitrites in food because
A)they produce nitrogen in the blood,causing a condition similar to the bends deep sea divers can suffer from.
B)as with ammonium nitrate fertilizers,they may cause small explosions in blood vessels causing them to rupture.
C)they interact with naturally occurring ozone in the body and remove it from the circulatory system.
D)they interact with the blood and limit its ability to transport oxygen.
A)they produce nitrogen in the blood,causing a condition similar to the bends deep sea divers can suffer from.
B)as with ammonium nitrate fertilizers,they may cause small explosions in blood vessels causing them to rupture.
C)they interact with naturally occurring ozone in the body and remove it from the circulatory system.
D)they interact with the blood and limit its ability to transport oxygen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
48
The attractions between anions and cations throughout a crystal are known collectively as
A)covalent bonds.
B)polar covalent bonds.
C)hydrogen bonds.
D)ionic bonds.
A)covalent bonds.
B)polar covalent bonds.
C)hydrogen bonds.
D)ionic bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which ions,if present in drinking water,would pose a significant health risk?
i.Pb2+
II.Hg2+
III.Cd2+
IV.Ca2+
A)I,II,and III only
B)II,III,and IV only
C)I,III,and IV only
D)I,II,III,and IV
i.Pb2+
II.Hg2+
III.Cd2+
IV.Ca2+
A)I,II,and III only
B)II,III,and IV only
C)I,III,and IV only
D)I,II,III,and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
50
The main reason that water supplies are "chlorinated" is
A)to produce gels that remove solids from the water.
B)to kill disease-causing organisms in the water.
C)to soften the water.
D)to precipitate lead salts from the water as insoluble lead chloride.
A)to produce gels that remove solids from the water.
B)to kill disease-causing organisms in the water.
C)to soften the water.
D)to precipitate lead salts from the water as insoluble lead chloride.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
51
Reverse osmosis is illustrated in this diagram.What is this process used for? 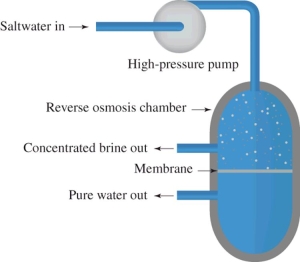
A)to purify water by ion exchange
B)to remove dissolved salts from sea water
C)for the ozonation of water supplies
D)to add fluorine to municipal water supplies

A)to purify water by ion exchange
B)to remove dissolved salts from sea water
C)for the ozonation of water supplies
D)to add fluorine to municipal water supplies

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which compound is insoluble in water?
A)sodium carbonate
B)potassium nitrate
C)ammonium chloride
D)calcium carbonate
A)sodium carbonate
B)potassium nitrate
C)ammonium chloride
D)calcium carbonate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which form of water disinfection continues to provide antibacterial protection after the water leaves the purification plant?
A)chlorination
B)ozonation
C)UV radiation
D)filtration
A)chlorination
B)ozonation
C)UV radiation
D)filtration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
54
We cannot effectively clean nonpolar substances from our hands or clothing with water alone;we must add soap or detergent.The structure of a typical soap molecule is shown below.Which region of this molecule would dissolve in a nonpolar substance such as grease? 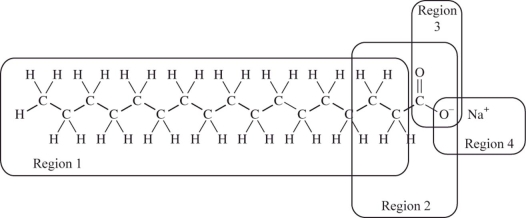
A)Region 1
B)Region 2
C)Region 3
D)Region 4

A)Region 1
B)Region 2
C)Region 3
D)Region 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which compounds are not soluble in water at room temperature? 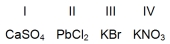
A)I and II only
B)II and III only
C)III and IV only
D)I and IV only
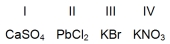
A)I and II only
B)II and III only
C)III and IV only
D)I and IV only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
56
The steps in the typical purification processes for municipal drinking water are,in order,
A)filtration,distillation,chlorination,and filtration.
B)filtration,chlorination,ozonation,and flocculation.
C)filtration,flocculation,filtration,and chlorination.
D)flocculation,filtration,UV irradiation,and filtration.
A)filtration,distillation,chlorination,and filtration.
B)filtration,chlorination,ozonation,and flocculation.
C)filtration,flocculation,filtration,and chlorination.
D)flocculation,filtration,UV irradiation,and filtration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which compound should be the most soluble in water?
A)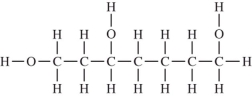
B)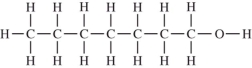
C)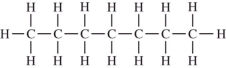
D)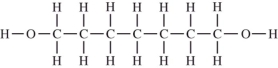
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which statement is not correct?
A)Lead has been used in water pipes since ancient times because it is an abundant,soft,and easily worked metal that does not rust.
B)The symbol for lead,Pb,comes from the Latin name for lead "plumbum".
C)When ingested,lead causes severe and permanent neurological problems in humans.
D)The EPA estimates that one in two U.S.children under six years of age has a blood lead level above the acceptable level of 15 g/mL.
A)Lead has been used in water pipes since ancient times because it is an abundant,soft,and easily worked metal that does not rust.
B)The symbol for lead,Pb,comes from the Latin name for lead "plumbum".
C)When ingested,lead causes severe and permanent neurological problems in humans.
D)The EPA estimates that one in two U.S.children under six years of age has a blood lead level above the acceptable level of 15 g/mL.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
59
What are the major disadvantages of using ozone instead of chlorine to disinfect water?
A)Ozonation is more expensive than chlorination and ozone leaves an odor in the water.
B)Ozonation causes trihalomethane formation and leaves an odor in the treated water.
C)Ozonation causes trihalomethane formation and is more expensive than chlorination.
D)Ozone does not provide long-term protection against waterborne viruses because it decomposes quickly and it is more expensive than chlorination.
A)Ozonation is more expensive than chlorination and ozone leaves an odor in the water.
B)Ozonation causes trihalomethane formation and leaves an odor in the treated water.
C)Ozonation causes trihalomethane formation and is more expensive than chlorination.
D)Ozone does not provide long-term protection against waterborne viruses because it decomposes quickly and it is more expensive than chlorination.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
60
A disadvantage of ozonation over chlorination is
A)the higher cost of ozonation.
B)ozonation does not protect the water after the initial process is complete.
C)the higher cost of ozonation and ozonation does not protect the water after the initial process is complete.
D)None of these choices is correct.
A)the higher cost of ozonation.
B)ozonation does not protect the water after the initial process is complete.
C)the higher cost of ozonation and ozonation does not protect the water after the initial process is complete.
D)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the following are methods of making sea water appropriate for drinking?
__ Osmosis
__ Reverse osmosis
__ Distillation
__ Flocculation
__ Osmosis
__ Reverse osmosis
__ Distillation
__ Flocculation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is biological oxygen demand (BOD)?
A)A measure of the amount of dissolved oxygen microorganisms use up as they decompose organic wastes found in water.
B)A measure of the amount of dissolved oxygen necessary to purify wastewater.
C)A measure of the phosphate ion concentration of water
D)A measure of nitrate ion concentration of water.
A)A measure of the amount of dissolved oxygen microorganisms use up as they decompose organic wastes found in water.
B)A measure of the amount of dissolved oxygen necessary to purify wastewater.
C)A measure of the phosphate ion concentration of water
D)A measure of nitrate ion concentration of water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which compound has an incorrect formula?
A)MgPO4
B)(NH4)2S
C)Mg(HSO3)2
D)NaClO4
A)MgPO4
B)(NH4)2S
C)Mg(HSO3)2
D)NaClO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
64
The world map shows the availability of safe drinking water around the world.Which statement is true? 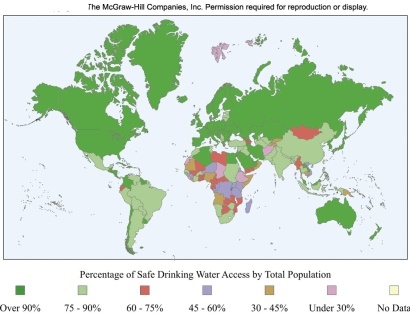
A)In Australia,less than 75% of the population has access to safe drinking water.
B)In most of Africa,more than 90% of the population has access to clean water.
C)In Europe and Russia,more than 10% of the population does not have access to clean water.
D)In the United States and Canada,more than 90% of the population has access to clean water.

A)In Australia,less than 75% of the population has access to safe drinking water.
B)In most of Africa,more than 90% of the population has access to clean water.
C)In Europe and Russia,more than 10% of the population does not have access to clean water.
D)In the United States and Canada,more than 90% of the population has access to clean water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which compound has a correct formula?
A)CaSO3
B)Ca2SO4
C)Mg(CO3)2
D)Na2PO4
A)CaSO3
B)Ca2SO4
C)Mg(CO3)2
D)Na2PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
66
The drawing shows a simple way of purifying salt water.What is this process called? 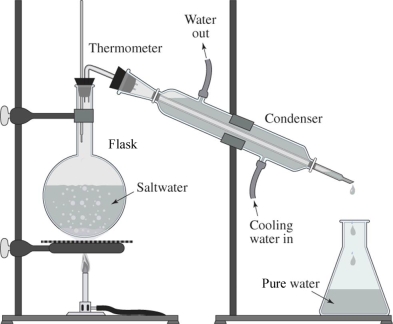
A)filtration
B)distillation
C)ozonation
D)chlorination

A)filtration
B)distillation
C)ozonation
D)chlorination

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
67
A 2- ion has 36 electrons and 45 neutrons.What is its correct symbol?
A)(83Sr2-)
B)(71Br2-)
C)(79Se 2-)
D)(78As 2-)
A)(83Sr2-)
B)(71Br2-)
C)(79Se 2-)
D)(78As 2-)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
68
How many bromide ions will combine with one ion of aluminum?
A)3
B)6
C)1
D)2
A)3
B)6
C)1
D)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
69
Why is water a liquid a room temperature instead of a gas?
A)It is a gas a room temperature.
B)Water has hydrogen bonds between the molecules.
C)Water is very acidic.
D)Water is a good solvent.
A)It is a gas a room temperature.
B)Water has hydrogen bonds between the molecules.
C)Water is very acidic.
D)Water is a good solvent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following has the highest MCL in drinking water in the US?
A)Nitrates
B)Trihalomethanes
C)Lead
D)Mercury
A)Nitrates
B)Trihalomethanes
C)Lead
D)Mercury

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following is not a consequence of biomagnification?
A)Consumption of tuna should be limited.
B)DDT from fish was killing large birds before the pesticide was banned.
C)Lead in water can lead to brain damage in children.
D)Polar compounds are found in high concentrations in predator animals.
A)Consumption of tuna should be limited.
B)DDT from fish was killing large birds before the pesticide was banned.
C)Lead in water can lead to brain damage in children.
D)Polar compounds are found in high concentrations in predator animals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
72
Mixing which of the following will produce a precipitation reaction (give an insoluble product)?
A)HNO3 (aq)and Sr(OH)2(aq)
B)LiNO3 and NaI
C)Na2SO4(aq)and Ba(OH)2(aq)
D)NaOH and KBr
A)HNO3 (aq)and Sr(OH)2(aq)
B)LiNO3 and NaI
C)Na2SO4(aq)and Ba(OH)2(aq)
D)NaOH and KBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
73
The MCL for drinking water is the legal limit for the concentration of a contaminant expressed in ppm or ppb.This is always a lower concentration than the MCLG value for the same contaminant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which type of water may be considered pure?
A)bottled water
B)filtered water
C)distilled water
D)chlorinated water
A)bottled water
B)filtered water
C)distilled water
D)chlorinated water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck








