Deck 12: Liquids, solids, and Intermolecular Forces
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/115
العب
ملء الشاشة (f)
Deck 12: Liquids, solids, and Intermolecular Forces
1
The rate of evaporation will increase if you pour a liquid out onto a large surface.
True
2
Evaporation is decreased by increasing the intermolecular forces.
True
3
Compounds with very high vapor pressures must have very minimal intermolecular forces.
True
4
Without intermolecular forces,solids and liquids would not exist and all matter would be gaseous.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
5
Atoms in a solid are always arranged in a well-ordered pattern.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
6
Liquids that are viscous flow more slowly than liquids that are not viscous.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
7
Viscosity increases with increased intermolecular force because the molecules attract each other strongly which hinders the flow.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
8
The process of heating a pot of water from room temperature to boiling temperature is an exothermic process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
9
Intermolecular forces are the attractive forces between atoms within a compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
10
Evaporation and condensation are opposite processes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
11
Solids usually have much greater densities than gases because molecules of a solid are much farther apart.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
12
The boiling point is the temperature at which the vapor pressure of a solution is equal to the intermolecular forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
13
Liquids can be easily compressed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
14
Evaporation is an endothermic process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
15
Oil would be considered a nonvolatile liquid while gasoline would be considered volatile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
16
Gases typically have high densities in comparison to solids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
17
The strength of the surface tension is inversely related to the strength of the intermolecular forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
18
Increasing the temperature increases the vaporization rate of a liquid because the excess energy is used to break covalent bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
19
The shapes of protein molecules are determined by intermolecular forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
20
Intermolecular forces determine if a substance is a solid,liquid or gas at room temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
21
Water has a heat of vaporization of 44.0 kJ/mol while the chemical diethyl ether has a heat of vaporization of 27.1 kJ/mol.This shows that one mole of water would vaporize more easily than would one mole of diethyl ether.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
22
Ice is an example of a molecular solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
23
Heat is released when a liquid freezes into a solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
24
All intermolecular forces are broken when a liquid vaporizes into a gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
25
Intermolecular forces hold atoms and molecules in place in a solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
26
H2 boils at a higher temperature than He because H2 undergoes hydrogen bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
27
Dipole-dipole forces are weaker than dispersion forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
28
Ion-dipole forces occur in mixtures of ionic compounds and polar compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
29
Dispersion forces result from the temporary distortion of the electron cloud in an atom or molecule which increases in magnitude with increasing size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
30
The heat of fusion for water is significantly more than the heat of vaporization for water because fusion requires complete separation of one molecule from another.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
31
A glass of ice water containing nine ice cubes will be colder than a similar-size glass of ice water containing only three ice cubes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
32
Atomic solids,such as graphite,have a weak dispersion force holding them together.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
33
Ion-dipole force is considered the weakest of the different types of intermolecular forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
34
For hydrogen bonding to occur,a molecule must have a hydrogen atom bonded directly to a fluorine,oxygen,or nitrogen atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
35
Ionic solids tend to have higher melting points than molecular solids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
36
When one mole of water vapor at 100°C condenses,it will release an amount of energy equivalent to its heat of vaporization (40.7 kJ).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
37
Sublimation is the process of a liquid being converted directly to a gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
38
Attraction between the Na+ cation and Cl- anion holds the solid lattice of sodium chloride together.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
39
The melting point is reached when sufficient energy has been added to the molecules in a substance to overcome the intermolecular forces holding them stationary.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
40
Hydrogen bonding occurs whenever hydrogen is present.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
41
Compounds that are similar to water in molecular mass all exist as solids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
42
Increasing the intermolecular forces of a liquid will do which of the following?
A)increase the viscosity
B)decrease the evaporation rate
C)increase the surface tension
D)decrease the vapor pressure
E)all of the above
A)increase the viscosity
B)decrease the evaporation rate
C)increase the surface tension
D)decrease the vapor pressure
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
43
The tendency of a liquid to minimize its surface area is called:
A)capillary action.
B)viscosity.
C)surface tension.
D)vaporization.
E)none of the above
A)capillary action.
B)viscosity.
C)surface tension.
D)vaporization.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
44
The change of a substance from a liquid to a gaseous form is called:
A)dynamic equilibrium.
B)heat of fusion.
C)condensation.
D)vaporization.
E)volatile.
A)dynamic equilibrium.
B)heat of fusion.
C)condensation.
D)vaporization.
E)volatile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which state of matter has a low density and is easily compressed?
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
46
Evaporation is:
A)increased by increasing temperature.
B)an endothermic process.
C)the opposite process to condensation.
D)a cooling process for humans when they sweat.
E)all of the above
A)increased by increasing temperature.
B)an endothermic process.
C)the opposite process to condensation.
D)a cooling process for humans when they sweat.
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which statement about surface tension is FALSE?
A)Liquids tend to minimize their surface area.
B)Molecules on the surface of the liquid have fewer molecules to interact with.
C)Increased intermolecular forces increase surface tension.
D)Items with densities lower than water will sink due to surface tension.
E)All of the above statements are true.
A)Liquids tend to minimize their surface area.
B)Molecules on the surface of the liquid have fewer molecules to interact with.
C)Increased intermolecular forces increase surface tension.
D)Items with densities lower than water will sink due to surface tension.
E)All of the above statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which state of matter has a low density and an indefinite volume?
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
49
The rate of vaporization of a liquid can be increased by:
1.increasing the surface area
2.increasing the temperature
3.increasing the strength of the intermolecular forces
A)1 only
B)2 only
C)3 only
D)1 and 2 only
E)2 and 3 only
1.increasing the surface area
2.increasing the temperature
3.increasing the strength of the intermolecular forces
A)1 only
B)2 only
C)3 only
D)1 and 2 only
E)2 and 3 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which state of matter has a high density and a definite volume?
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
51
The measure of the resistance to the flow of a liquid is called:
A)vapor pressure.
B)sublimation.
C)viscosity.
D)condensation.
E)none of the above
A)vapor pressure.
B)sublimation.
C)viscosity.
D)condensation.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
52
The opposite process of freezing is:
A)evaporation.
B)sublimation.
C)boiling.
D)condensation.
E)none of the above
A)evaporation.
B)sublimation.
C)boiling.
D)condensation.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
53
A situation where two opposite processes are occurring at equal rates,and no net change is taking place,is called:
A)vaporization.
B)condensation.
C)evaporation.
D)dynamic equilibrium.
E)none of the above
A)vaporization.
B)condensation.
C)evaporation.
D)dynamic equilibrium.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
54
Ice can float in a glass of liquid water because the solid form of water is more dense than the liquid form.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
55
You can increase the vapor pressure of a liquid by:
A)increasing temperature.
B)increasing the viscosity.
C)establishing dynamic equilibrium.
D)using a nonvolatile compound.
E)all of the above
A)increasing temperature.
B)increasing the viscosity.
C)establishing dynamic equilibrium.
D)using a nonvolatile compound.
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which statement about boiling point is FALSE?
A)The boiling point is higher for compounds with strong intermolecular forces.
B)The boiling point is higher for compounds with a high viscosity.
C)The boiling point of a compound is an absolute constant.
D)The boiling point of a compound is higher for nonvolatile compounds.
E)All of the above statements are true.
A)The boiling point is higher for compounds with strong intermolecular forces.
B)The boiling point is higher for compounds with a high viscosity.
C)The boiling point of a compound is an absolute constant.
D)The boiling point of a compound is higher for nonvolatile compounds.
E)All of the above statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
57
Water has unusual physical properties which enable life to exist on earth.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of the following statements is false?
A)Evaporation is an endothermic process.
B)A puddle of water cools down as it evaporates.
C)As a liquid is converted into a gas,the liquid absorbs heat.
D)All of the above are true.
E)None of the above are true.
A)Evaporation is an endothermic process.
B)A puddle of water cools down as it evaporates.
C)As a liquid is converted into a gas,the liquid absorbs heat.
D)All of the above are true.
E)None of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which state of matter has a high density and an indefinite shape?
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above
A)solids
B)liquids
C)gases
D)both solids and liquids
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
60
Intermolecular forces are responsible for:
A)the taste sensations.
B)the shape of protein molecules.
C)the function of DNA.
D)the existence of liquids and solids.
E)all of the above
A)the taste sensations.
B)the shape of protein molecules.
C)the function of DNA.
D)the existence of liquids and solids.
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
61
How much energy does it take to melt a 16.87 g ice cube?
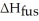 = 6.02 kJ/mol
= 6.02 kJ/mol
A)102 kJ
B)108 kJ
C)936 J
D)5.64 kJ
E)none of the above
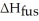 = 6.02 kJ/mol
= 6.02 kJ/molA)102 kJ
B)108 kJ
C)936 J
D)5.64 kJ
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
62
The ability of sodium chloride to mix with water is most likely due to:
A)dispersion force
B)ion-dipole force
C)dipole-dipole force
D)hydrogen bonding
E)none of the above
A)dispersion force
B)ion-dipole force
C)dipole-dipole force
D)hydrogen bonding
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the heat of vaporization(kJ/mol)if it takes 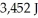 of heat to completely vaporize
of heat to completely vaporize 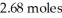 of the liquid at its boiling point?
of the liquid at its boiling point?
A)1288
B)1.29
C)0.776
D)12.2
E)none of the above
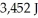 of heat to completely vaporize
of heat to completely vaporize 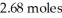 of the liquid at its boiling point?
of the liquid at its boiling point?A)1288
B)1.29
C)0.776
D)12.2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
64
The amount of heat required to melt one mole of a solid is called the:
A)heat of vaporization.
B)heat of fusion.
C)heating curve.
D)cooling curve.
E)none of the above
A)heat of vaporization.
B)heat of fusion.
C)heating curve.
D)cooling curve.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
65
How many kilojoules of heat are needed to completely vaporize 428 grams of C4H10O at its boiling point?
Given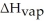 = 26.5kJ/mol
= 26.5kJ/mol
A)74.12
B)9.49
C)15.3
D)16.3
E)none of the above
Given
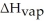 = 26.5kJ/mol
= 26.5kJ/molA)74.12
B)9.49
C)15.3
D)16.3
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
66
Gaseous water vapor can frost the windows of a car on a cold morning.This process of a gas changing directly into a solid is known as:
A)deposition.
B)melting.
C)condensation.
D)sublimation.
E)none of the above
A)deposition.
B)melting.
C)condensation.
D)sublimation.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which substance below has dipole-dipole forces?
A)CH4
B)CO2
C)F2
D)H2S
E)none of the above
A)CH4
B)CO2
C)F2
D)H2S
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
68
Liquids that have high vapor pressure and low boiling points are called:
A)abnormal liquids.
B)volatile liquids.
C)non-volatile liquids.
D)viscous liquids.
E)none of the above
A)abnormal liquids.
B)volatile liquids.
C)non-volatile liquids.
D)viscous liquids.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which statement is TRUE in describing what occurs when a solid melts to a liquid?
A)The process is endothermic and the heat of fusion is positive.
B)The process is endothermic and the heat of fusion is negative.
C)The process is exothermic and the heat of fusion is positive.
D)The process is exothermic and the heat of fusion is negative.
E)not enough information
A)The process is endothermic and the heat of fusion is positive.
B)The process is endothermic and the heat of fusion is negative.
C)The process is exothermic and the heat of fusion is positive.
D)The process is exothermic and the heat of fusion is negative.
E)not enough information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
70
When sufficient quantity of heat has been added to reach the boiling point of a solution,what happens to any additional heat added?
A)Additional heat is used to evaporate the liquid as the process is endothermic and requires continued input of energy.
B)Additional heat raises the temperature of the liquid which in turn increases the rate at which boiling occurs.
C)Additional heat lowers the intermolecular forces of the liquid which in turn increases the volatility of the liquid.
D)Additional heat alters the viscosity and the surface tension of the liquid which raises the vapor pressure and increases the boiling point which is why you must continually heat the solution.
E)None of the above are correct statements.
A)Additional heat is used to evaporate the liquid as the process is endothermic and requires continued input of energy.
B)Additional heat raises the temperature of the liquid which in turn increases the rate at which boiling occurs.
C)Additional heat lowers the intermolecular forces of the liquid which in turn increases the volatility of the liquid.
D)Additional heat alters the viscosity and the surface tension of the liquid which raises the vapor pressure and increases the boiling point which is why you must continually heat the solution.
E)None of the above are correct statements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which intermolecular force is due to the formation of an instantaneous dipole?
A)dispersion forces
B)dipole-dipole forces
C)hydrogen bonding
D)X-forces
E)none of the above
A)dispersion forces
B)dipole-dipole forces
C)hydrogen bonding
D)X-forces
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
72
When you make ice cubes:
A)it is an endothermic process.
B)it is an exothermic process.
C)the heat of vaporization must be removed.
D)the process is referred to scientifically as sublimation.
E)none of the above
A)it is an endothermic process.
B)it is an exothermic process.
C)the heat of vaporization must be removed.
D)the process is referred to scientifically as sublimation.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
73
In northern climates,it is common to have a layer of frost form on cars that have been out overnight in the winter.During the day the frost layer disappears despite the temperature of the ice remaining below freezing.How?
A)The frost melts due to the sun heating the surface of the car above the melting point.
B)The frost evaporates due to the sun heating the solid.
C)The frost cycles as does the saturation level of moisture in the winter air does from night to day.
D)The frost sublimes directly from solid ice to water vapor.
E)none of the above
A)The frost melts due to the sun heating the surface of the car above the melting point.
B)The frost evaporates due to the sun heating the solid.
C)The frost cycles as does the saturation level of moisture in the winter air does from night to day.
D)The frost sublimes directly from solid ice to water vapor.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
74
Paradichlorobenzene,a material used in "moth balls," is known to go directly from a solid form to a gaseous form.This process is known as:
A)melting
B)evaporation
C)condensation
D)boiling
E)sublimation
A)melting
B)evaporation
C)condensation
D)boiling
E)sublimation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
75
Compare a small pot of water that is boiling vigorously to a large pot of water that is boiling gently.Which statement is TRUE?
A)The small pot is boiling at higher temperature than the large pot.
B)The large pot is boiling at a higher temperature than the small pot.
C)Both pots are boiling at the same temperature.
D)The vapor pressure of the liquid is greater than the pressure above the pot in each case.
E)none of the above
A)The small pot is boiling at higher temperature than the large pot.
B)The large pot is boiling at a higher temperature than the small pot.
C)Both pots are boiling at the same temperature.
D)The vapor pressure of the liquid is greater than the pressure above the pot in each case.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
76
How many joules of heat are needed to completely vaporize 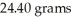 of water at its boiling point? Given
of water at its boiling point? Given 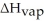 = 40.6 kJ/mol
= 40.6 kJ/mol
A)54.97
B)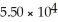
C)29.98
D)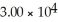
E)none of the above
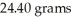 of water at its boiling point? Given
of water at its boiling point? Given 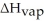 = 40.6 kJ/mol
= 40.6 kJ/molA)54.97
B)
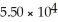
C)29.98
D)
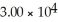
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which intermolecular force is present in all molecules and atoms?
A)dispersion forces
B)dipole-dipole forces
C)hydrogen bonding
D)X-forces
E)none of the above
A)dispersion forces
B)dipole-dipole forces
C)hydrogen bonding
D)X-forces
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
78
What happens as you start to add heat to a solid substance?
A)Thermal energy causes the components of the solid to vibrate faster.
B)When the melting point is reached,the thermal energy is sufficient to overcome intermolecular forces holding the components at their stationary points.
C)Increasing the rate of heating of a substance at its melting point only causes more rapid melting.
D)If a liquid forms,continued heating results in increasing the liquid temperature.
E)all of the above
A)Thermal energy causes the components of the solid to vibrate faster.
B)When the melting point is reached,the thermal energy is sufficient to overcome intermolecular forces holding the components at their stationary points.
C)Increasing the rate of heating of a substance at its melting point only causes more rapid melting.
D)If a liquid forms,continued heating results in increasing the liquid temperature.
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
79
How many grams of C4H10O can be melted by 2.00 X 103 J?
Given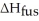 =7.27 kJ/mol
=7.27 kJ/mol
A)14.5
B)3.64
C)20.4
D)74.1
E)none of the above
Given
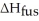 =7.27 kJ/mol
=7.27 kJ/molA)14.5
B)3.64
C)20.4
D)74.1
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
80
If we supply additional heat to a solid in equilibrium with its liquid at the melting point,the thermal energy added is used to:
A)overcome the intermolecular forces that hold the solid together.
B)expand the solid.
C)change the liquid back to solid.
D)change solid to liquid.
E)raise the temperature of the solid above its melting point.
A)overcome the intermolecular forces that hold the solid together.
B)expand the solid.
C)change the liquid back to solid.
D)change solid to liquid.
E)raise the temperature of the solid above its melting point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck








