Deck 9: The Atom
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/46
العب
ملء الشاشة (f)
Deck 9: The Atom
1
Photons have
A)no charge.
B)charges smaller than e, like quarks.
C)charges of e.
D)charges whose magnitudes depends on their speeds.
A)no charge.
B)charges smaller than e, like quarks.
C)charges of e.
D)charges whose magnitudes depends on their speeds.
no charge.
2
In order to increase the rate at which photoelectrons are emitted by a surface on which light is directed, it is necessary to increase the
A)frequency of the light.
B)wavelength of the light.
C)intensity of the light.
D)speed of the light.
A)frequency of the light.
B)wavelength of the light.
C)intensity of the light.
D)speed of the light.
intensity of the light.
3
According to the uncertainty principle, it is impossible to precisely determine
A)only the position of a particle.
B)only the momentum of a particle.
C)either the position or the momentum of a particle.
D)both the position and the momentum of a particle.
A)only the position of a particle.
B)only the momentum of a particle.
C)either the position or the momentum of a particle.
D)both the position and the momentum of a particle.
both the position and the momentum of a particle.
4
The narrower the wave packet of a particle is, the
A)longer its wavelength.
B)more precisely its position can be established.
C)more precisely its momentum can be established.
D)more precisely its energy can be established.
A)longer its wavelength.
B)more precisely its position can be established.
C)more precisely its momentum can be established.
D)more precisely its energy can be established.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
5
A proton and an electron have the same de Broglie wavelength.
A)The electron has the higher speed.
B)The proton has the higher speed.
C)They have the same speed.
D)Any of the choices is true, depending on the wavelength.
A)The electron has the higher speed.
B)The proton has the higher speed.
C)They have the same speed.
D)Any of the choices is true, depending on the wavelength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
6
A moving body is described by its wave function 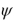 at a certain time and place.The value of
at a certain time and place.The value of 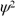 is proportional to the body's
is proportional to the body's
A)electric field.
B)speed.
C)energy.
D)probability of being found.
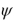 at a certain time and place.The value of
at a certain time and place.The value of 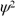 is proportional to the body's
is proportional to the body'sA)electric field.
B)speed.
C)energy.
D)probability of being found.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
7
X-rays are
A)electromagnetic waves of very short wavelength.
B)electromagnetic waves of very long wavelength.
C)matter waves of very short wavelength.
D)matter waves of very long wavelength.
A)electromagnetic waves of very short wavelength.
B)electromagnetic waves of very long wavelength.
C)matter waves of very short wavelength.
D)matter waves of very long wavelength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
8
If Planck's constant were larger than it is,
A)moving bodies would have longer wavelengths.
B)moving bodies would have smaller energies.
C)moving bodies would have higher speeds.
D)the uncertainty principle would be more significant.
A)moving bodies would have longer wavelengths.
B)moving bodies would have smaller energies.
C)moving bodies would have higher speeds.
D)the uncertainty principle would be more significant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
9
The idea that light has both wave and particle properties is accepted because
A)theory and experiment agree.
B)it is in accord with common sense.
C)it was revealed to Einstein in a dream.
D)photons have actually been seen.
A)theory and experiment agree.
B)it is in accord with common sense.
C)it was revealed to Einstein in a dream.
D)photons have actually been seen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
10
An electron and a gamma ray have the same wavelength.
A)The electron has less energy than the photon energy of the gamma ray.
B)The electron has the same energy as the photon energy of the gamma ray.
C)The electron has more energy than the photon energy of the gamma ray.
D)Any of these choices could be correct, depending on the wavelength.
A)The electron has less energy than the photon energy of the gamma ray.
B)The electron has the same energy as the photon energy of the gamma ray.
C)The electron has more energy than the photon energy of the gamma ray.
D)Any of these choices could be correct, depending on the wavelength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
11
The uncertainty principle is a fundamental part of
A)Newtonian mechanics.
B)quantum mechanics.
C)the quantum theory of light.
D)the Bohr theory of the hydrogen atom.
A)Newtonian mechanics.
B)quantum mechanics.
C)the quantum theory of light.
D)the Bohr theory of the hydrogen atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
12
Matter waves are
A)associated with all particles.
B)never associated with stationary particles.
C)never associated with charged particles.
D)never associated with uncharged particles.
A)associated with all particles.
B)never associated with stationary particles.
C)never associated with charged particles.
D)never associated with uncharged particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
13
When light is directed at a metal surface, the emitted electrons
A)are called photons.
B)have random energies.
C)have energies that depend upon the intensity of the light.
D)have energies that depend upon the frequency of the light.
A)are called photons.
B)have random energies.
C)have energies that depend upon the intensity of the light.
D)have energies that depend upon the frequency of the light.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which one or more of the following can be explained by the quantum theory of light?
A)interference
B)diffraction
C)the photoelectric effect
D)matter waves
A)interference
B)diffraction
C)the photoelectric effect
D)matter waves

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
15
The wave character of a moving golf ball is not observed because
A)the ball is uncharged.
B)the matter waves are moving too fast.
C)the frequency of the matter waves is too low.
D)the wavelength of the matter waves is too short.
A)the ball is uncharged.
B)the matter waves are moving too fast.
C)the frequency of the matter waves is too low.
D)the wavelength of the matter waves is too short.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
16
An increase in the voltage applied to an X-ray tube causes an increase in the X-rays'
A)wavelength.
B)speed.
C)energy.
D)number.
A)wavelength.
B)speed.
C)energy.
D)number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
17
The photoelectric effect occurs only when the incident light has more than a certain minimum
A)frequency.
B)wavelength.
C)speed.
D)charge.
A)frequency.
B)wavelength.
C)speed.
D)charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
18
The speed of the wave packet that corresponds to a moving particle is
A)less than the speed of light.
B)equal to the speed of light.
C)greater than the speed of light.
D)Any of these choices could be correct, depending on the frequency.
A)less than the speed of light.
B)equal to the speed of light.
C)greater than the speed of light.
D)Any of these choices could be correct, depending on the frequency.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which one or more of the following can be explained by the electromagnetic theory of light?
A)interference
B)diffraction
C)the photoelectric effect
D)matter waves
E)Both A and B
A)interference
B)diffraction
C)the photoelectric effect
D)matter waves
E)Both A and B

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
20
The speed of a photon
A)is less than the speed of light.
B)is equal to the speed of light.
C)is greater than the speed of light.
D)Any of these choices could be correct, depending on its frequency.
A)is less than the speed of light.
B)is equal to the speed of light.
C)is greater than the speed of light.
D)Any of these choices could be correct, depending on its frequency.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
21
Classical physics cannot account for why
A)atoms can contain electrons.
B)atomic electrons are in motion.
C)atoms can emit electromagnetic waves.
D)atoms can be stable.
A)atoms can contain electrons.
B)atomic electrons are in motion.
C)atoms can emit electromagnetic waves.
D)atoms can be stable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following electron jumps in a hydrogen atom emits the photon of lowest frequency?
A)n = 1 to n = 2
B)n = 2 to n = 1
C)n = 2 to n = 3
D)n = 3 to n = 2
A)n = 1 to n = 2
B)n = 2 to n = 1
C)n = 2 to n = 3
D)n = 3 to n = 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
23
The bright-line spectrum produced by the excited atoms of an element contains wavelengths that are
A)the same for all elements.
B)characteristic of the particular element.
C)evenly distributed throughout the entire visible spectrum.
D)different from the wavelengths in its dark-line spectrum.
A)the same for all elements.
B)characteristic of the particular element.
C)evenly distributed throughout the entire visible spectrum.
D)different from the wavelengths in its dark-line spectrum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
24
The fact that no two electrons in an atom can have the same set of quantum numbers is known as the
A)Bohr principle.
B)de Broglie principle.
C)exclusion principle.
D)uncertainty principle.
A)Bohr principle.
B)de Broglie principle.
C)exclusion principle.
D)uncertainty principle.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
25
An atom in an excited state usually emits a photon and falls to a state of lower energy m about
A)10-8 s.
B)1 s.
C)10 s.
D)1 min.
A)10-8 s.
B)1 s.
C)10 s.
D)1 min.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
26
The quantum number n of the lowest energy state of a hydrogen atom
A)is 0.
B)is 1.
C)depends on the orbit size.
D)depends on the electron speed.
A)is 0.
B)is 1.
C)depends on the orbit size.
D)depends on the electron speed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
27
When a hydrogen atom is in its lowest energy level,
A)the de Broglie wavelength of its electron equals its orbit diameter.
B)the de Broglie wavelength of its electron equals its orbit circumference.
C)its electron has left the atom.
D)its electron is at rest.
A)the de Broglie wavelength of its electron equals its orbit diameter.
B)the de Broglie wavelength of its electron equals its orbit circumference.
C)its electron has left the atom.
D)its electron is at rest.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which one or more of the following events can raise an atom from its ground state to an excited state?
A)spontaneous emission of a photon
B)induced emission of a photon
C)absorption of a photon
D)a collision with another atom
E)Both C and D
A)spontaneous emission of a photon
B)induced emission of a photon
C)absorption of a photon
D)a collision with another atom
E)Both C and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
29
A beam of light whose waves are all in step with one another is said to be
A)coherent.
B)incoherent.
C)quantized.
D)diffracted.
A)coherent.
B)incoherent.
C)quantized.
D)diffracted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following electron jumps in a hydrogen atom absorbs the photon of highest frequency?
A)n = 1 to n = 2
B)n = 2 to n = 1
C)n = 2 to n = 3
D)n = 3 to n = 2
A)n = 1 to n = 2
B)n = 2 to n = 1
C)n = 2 to n = 3
D)n = 3 to n = 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
31
A photon is emitted by an atom when one of the atom's electrons
A)leaves the atom.
B)collides with another of its electrons.
C)shifts to a lower energy level.
D)shifts to a higher energy level.
A)leaves the atom.
B)collides with another of its electrons.
C)shifts to a lower energy level.
D)shifts to a higher energy level.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
32
In an emission spectral series, the
A)lines are evenly spaced.
B)excited electrons all started from the same energy level.
C)excited electrons all return to the same energy level.
D)excited electrons leave the atoms.
A)lines are evenly spaced.
B)excited electrons all started from the same energy level.
C)excited electrons all return to the same energy level.
D)excited electrons leave the atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
33
The energy difference between adjacent energy levels in an atom
A)is the same for all quantum numbers.
B)is smaller for small quantum numbers.
C)is larger for large quantum numbers.
D)shows no regularity.
A)is the same for all quantum numbers.
B)is smaller for small quantum numbers.
C)is larger for large quantum numbers.
D)shows no regularity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
34
A neon sign does not produce
A)a line spectrum.
B)an emission spectrum.
C)an absorption spectrum.
D)photons.
A)a line spectrum.
B)an emission spectrum.
C)an absorption spectrum.
D)photons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
35
Most stars are hot objects surrounded by cooler atmospheres.The spectrum of such a star is a
A)continuous band of light.
B)band of light crossed by brighter lines.
C)band of light crossed by dark lines.
D)series of bright lines.
A)continuous band of light.
B)band of light crossed by brighter lines.
C)band of light crossed by dark lines.
D)series of bright lines.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
36
The light waves from a laser
A)have different wavelengths.
B)have different speeds.
C)are in step with one another.
D)spread out to form a wide beam.
A)have different wavelengths.
B)have different speeds.
C)are in step with one another.
D)spread out to form a wide beam.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
37
Electrons
A)have no magnetic properties.
B)have the same magnetic behavior as particles of iron.
C)behave like tiny bar magnets of different strengths.
D)behave like tiny bar magnets of the same strength.
A)have no magnetic properties.
B)have the same magnetic behavior as particles of iron.
C)behave like tiny bar magnets of different strengths.
D)behave like tiny bar magnets of the same strength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
38
The number of quantum numbers needed to determine the size and shape of the probability cloud of an atomic electron is
A)1.
B)2.
C)3.
D)4.
A)1.
B)2.
C)3.
D)4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
39
The number of values the spin magnetic quantum number ms of an atomic electron can have is
A)1.
B)2.
C)always equal to n, the principal quantum number.
D)always equal to 2n.
A)1.
B)2.
C)always equal to n, the principal quantum number.
D)always equal to 2n.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following electron jumps in a hydrogen atom emits the photon of highest frequency?
A)n = 1 to n = 2
B)n = 2 to n = 1
C)n = 2 to n = 3
D)n = 3 to n = 2
A)n = 1 to n = 2
B)n = 2 to n = 1
C)n = 2 to n = 3
D)n = 3 to n = 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
41
Planck's constant is 6.6 * 10-34 J·s and the proton mass is 1.7 * 10-27 kg.The speed of a proton whose de Broglie wavelength is 4.0 * 107 m is
A)7.8 * 10-20 m/s.
B)9.9 * 102 m/s.
C)1.9 * 106 m/s.
D)3.9 * 106 m/s.
A)7.8 * 10-20 m/s.
B)9.9 * 102 m/s.
C)1.9 * 106 m/s.
D)3.9 * 106 m/s.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
42
Planck's constant is h = 6.6 * 10-34 J·s.The energy of a photon of light whose frequency is 3 * 1014 Hz is
A)2.2 * 10-48 J.
B)1.98 * 10-47 J.
C)1.98 * 10-19 J.
D)4.5 * 1019 J.
A)2.2 * 10-48 J.
B)1.98 * 10-47 J.
C)1.98 * 10-19 J.
D)4.5 * 1019 J.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
43
Each probability cloud in an atom can be occupied by
A)only one electron.
B)two electrons with spins in the same direction.
C)two electrons with spins in opposite directions.
D)any number of electrons.
A)only one electron.
B)two electrons with spins in the same direction.
C)two electrons with spins in opposite directions.
D)any number of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
44
A lamp emits light of frequency 5 * 1015 Hz at a power of 25 W and Planck's constant is 6.6 * 10-34 J·s.The number of photons given off per second is
A)1.3 * 10-19.
B)8.3 * 10-17.
C)7.6 * 1018.
D)1.9 * 1050.
A)1.3 * 10-19.
B)8.3 * 10-17.
C)7.6 * 1018.
D)1.9 * 1050.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
45
Planck's constant is 6.6 * 10-34 J·s and the electron mass is 9.1 * 10-31 kg.The de Broglie wavelength of an electron whose speed is 2.0 * 107 m/s is
A)2.8 * 10-10 m.
B)3.6 * 10-10 m.
C)3.6 * 10-11 m.
D)2.8 * 1010 m.
A)2.8 * 10-10 m.
B)3.6 * 10-10 m.
C)3.6 * 10-11 m.
D)2.8 * 1010 m.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
46
An X-ray photon has an energy of 3.3 * 1015 J and Planck's constant is 6.6 * 10-34 J·s.The frequency that corresponds to this energy is
A)3.3 * 10-50 Hz.
B)3 * 10-19 Hz.
C)3.3 * 1018 Hz.
D)3.3 * 1020 Hz.
A)3.3 * 10-50 Hz.
B)3 * 10-19 Hz.
C)3.3 * 1018 Hz.
D)3.3 * 1020 Hz.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck








