Deck 3: Stoichiometry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/168
العب
ملء الشاشة (f)
Deck 3: Stoichiometry
1
What is the average mass, in grams, of one arsenic atom?
A)5.48 * 10-23 g
B)33.0 g
C)74.9 g
D)1.24 * 10-22 g
E)8.04 * 1021 g
A)5.48 * 10-23 g
B)33.0 g
C)74.9 g
D)1.24 * 10-22 g
E)8.04 * 1021 g
1.24 * 10-22 g
2
What is the mass of 7.80 * 1018 carbon atoms?
A)1.30 * 10 -5 g
B)6.43 * 103 g
C)7.80 * 1018 g
D)1.56 * 10 -4 g
E)12.01 g
A)1.30 * 10 -5 g
B)6.43 * 103 g
C)7.80 * 1018 g
D)1.56 * 10 -4 g
E)12.01 g
1.56 * 10 -4 g
3
Boron obtained from borax deposits in Death Valley consists of two isotopes. They are boron-10 and boron-11 with atomic masses of 10.013 amu and 11.009 amu, respectively. The atomic mass of boron is 10.81 amu (see periodic table). Which isotope of boron is more abundant, boron-10 or boron-11?
A)Cannot be determined from data given
B)Neither, their abundances are the same.
C)Boron-10
D)Boron-11
A)Cannot be determined from data given
B)Neither, their abundances are the same.
C)Boron-10
D)Boron-11
Boron-11
4
The element oxygen consists of three naturally occuring isotopes: 16O, 17O, and 18O. The atomic mass of oxygen is 16.0 amu. What can be implied about the relative abundances of these isotopes?
A)More than 50% of all O atoms are 17O.
B)Almost all O atoms are 18O.
C)Almost all O atoms are 17O.
D)The isotopes all have the same abundance, i.e. 33.3%.
E)The abundances of 17O and 18O are very small.
A)More than 50% of all O atoms are 17O.
B)Almost all O atoms are 18O.
C)Almost all O atoms are 17O.
D)The isotopes all have the same abundance, i.e. 33.3%.
E)The abundances of 17O and 18O are very small.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many silicon atoms are there in 1.00 g of silicon?
A)1 atom
B)0.0356 atoms
C)2.57 * 1023 atoms
D)2.14 * 1022 atoms
E)1.75 * 1025 atoms
A)1 atom
B)0.0356 atoms
C)2.57 * 1023 atoms
D)2.14 * 1022 atoms
E)1.75 * 1025 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
6
An average atom of uranium (U)is approximately how many times heavier than an atom of potassium?
A)6.1 times
B)4.8 times
C)2.4 times
D)12.5 times
E)7.7 times
A)6.1 times
B)4.8 times
C)2.4 times
D)12.5 times
E)7.7 times

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
7
Calculate the number of moles of xenon in 12.0 g of xenon.
A)1.00 mol
B)0.0457 mol
C)0.183 mol
D)7.62 * 10-3 mol
E)0.0914 mol
A)1.00 mol
B)0.0457 mol
C)0.183 mol
D)7.62 * 10-3 mol
E)0.0914 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which one of the following does not represent 1.00 mol of the indicated substance?
A)6.02 * 1023 C atoms
B)26.0 g Fe
C)12.01 g C
D)65.4 g Zn
E)6.02 * 1023 Fe atoms
A)6.02 * 1023 C atoms
B)26.0 g Fe
C)12.01 g C
D)65.4 g Zn
E)6.02 * 1023 Fe atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
9
Determine the number of moles of aluminum in 96.7 g of Al.
A)0.279 mol
B)3.58 mol
C)7.43 mol
D)4.21 mol
E)6.02 * 1023 mol
A)0.279 mol
B)3.58 mol
C)7.43 mol
D)4.21 mol
E)6.02 * 1023 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
10
If 0.274 moles of a substance weighs 62.5 g, what is the molar mass of the substance, in units of g/mol?
A)2.28 * 102 g/mol
B)1.71 * 101 g/mol
C)4.38 * 10 -3 g/mol
D)2.17 * 102 g/mol
E)none of these
A)2.28 * 102 g/mol
B)1.71 * 101 g/mol
C)4.38 * 10 -3 g/mol
D)2.17 * 102 g/mol
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
11
The mass of 1.21 *1020 atoms of sulfur is
A)3.88 * 1021 g.
B)2.00 mg.
C)32.06 g.
D)6.44 mg.
E)2.00 * 10-4 g.
A)3.88 * 1021 g.
B)2.00 mg.
C)32.06 g.
D)6.44 mg.
E)2.00 * 10-4 g.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
12
An atom of bromine has a mass about four times greater than that of an atom of neon. Which choice makes the correct comparison of the relative numbers of bromine and neon atoms in 1,000 g of each element?
A)The number of bromine and neon atoms is the same.
B)There are one thousand times as many bromine atoms as neon atoms.
C)There are one thousand times as many neon atoms as bromine atoms.
D)There are four times as many neon atoms as bromine atoms.
E)There are four times as many bromine atoms as neon atoms.
A)The number of bromine and neon atoms is the same.
B)There are one thousand times as many bromine atoms as neon atoms.
C)There are one thousand times as many neon atoms as bromine atoms.
D)There are four times as many neon atoms as bromine atoms.
E)There are four times as many bromine atoms as neon atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
13
An atom of helium has a mass about four times greater than that of an atom of hydrogen. Which choice makes the correct comparison of the relative numbers of helium and hydrogen atoms in equal masses of the two elements?
A)There are about four times as many helium atoms as hydrogen atoms.
B)There are about two times as many helium atoms as hydrogen atoms.
C)The number of helium and hydrogen atoms is the same.
D)There are about half as many helium atoms as hydrogen atoms.
E)There are about one-fourth as many helium atoms as hydrogen atoms.
A)There are about four times as many helium atoms as hydrogen atoms.
B)There are about two times as many helium atoms as hydrogen atoms.
C)The number of helium and hydrogen atoms is the same.
D)There are about half as many helium atoms as hydrogen atoms.
E)There are about one-fourth as many helium atoms as hydrogen atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
14
The mass of 1.63 * 1021 silicon atoms is
A)2.71 * 10-23 g.
B)4.58 * 1022 g.
C)28.08 g.
D)1.04 * 104 g.
E)7.60 * 10-2 g.
A)2.71 * 10-23 g.
B)4.58 * 1022 g.
C)28.08 g.
D)1.04 * 104 g.
E)7.60 * 10-2 g.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
15
An atom of bromine has a mass about four times greater than that of an atom of neon. How many grams of neon will contain the same number of atoms as 1,000 g of bromine?
A)4 g Ne
B)250 g Ne
C)400 g Ne
D)1,000 g Ne
E)4,000 g Ne
A)4 g Ne
B)250 g Ne
C)400 g Ne
D)1,000 g Ne
E)4,000 g Ne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
16
One mole of iron
A)is heavier than one mole of lead (Pb).
B)is 77.0 g of iron.
C)is 26.0 g of iron.
D)weighs the same as one mole of lead.
E)None of the above.
A)is heavier than one mole of lead (Pb).
B)is 77.0 g of iron.
C)is 26.0 g of iron.
D)weighs the same as one mole of lead.
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
17
One nanogram does not seem like a very large number. How many magnesium atoms are there in 1.00 ng of magnesium?
A)4.11 * 10-11 atoms
B)2.48 * 1013 atoms
C)6.83 * 10-35 atoms
D)6.02 * 1014 atoms
E)1.46 * 1034 atoms
A)4.11 * 10-11 atoms
B)2.48 * 1013 atoms
C)6.83 * 10-35 atoms
D)6.02 * 1014 atoms
E)1.46 * 1034 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
18
There are two stable isotopes of chlorine: chlorine-35, with a mass of 34.968853 amu; and chlorine-37, with a mass of 36.965903. Given that the average atomic mass of a chlorine atom is 35.45 amu, which of the following statements is true?
A)Chlorine contains almost exclusively of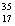 Cl, with very little
Cl, with very little 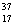 Cl.
Cl.
B)Chlorine contains more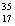 Cl than
Cl than 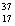 Cl.
Cl.
C)Chlorine contains roughly equal amounts of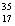 Cl and
Cl and 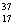 Cl.
Cl.
D)Chlorine contains more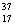 Cl than
Cl than 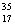 Cl.
Cl.
E)Chlorine contains almost exclusively of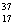 Cl, with very little
Cl, with very little 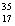 Cl.
Cl.
A)Chlorine contains almost exclusively of
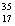 Cl, with very little
Cl, with very little 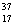 Cl.
Cl.B)Chlorine contains more
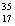 Cl than
Cl than 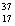 Cl.
Cl.C)Chlorine contains roughly equal amounts of
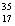 Cl and
Cl and 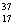 Cl.
Cl.D)Chlorine contains more
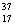 Cl than
Cl than 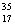 Cl.
Cl.E)Chlorine contains almost exclusively of
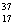 Cl, with very little
Cl, with very little 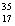 Cl.
Cl.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the average mass, in grams, of one potassium atom?
A)5.14 * 10-23 g
B)6.49 * 10-23 g
C)6.02 * 10-18 g
D)31.0 g
E)39.1 g
A)5.14 * 10-23 g
B)6.49 * 10-23 g
C)6.02 * 10-18 g
D)31.0 g
E)39.1 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the average mass, in grams, of one atom of iron?
A)6.02 * 1023 g
B)1.66 * 10-24 g
C)9.28 * 10-23 g
D)55.85 g
E)55.85 * 10 -23 g
A)6.02 * 1023 g
B)1.66 * 10-24 g
C)9.28 * 10-23 g
D)55.85 g
E)55.85 * 10 -23 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
21
A silver wire has a diameter of 0.500 mm. What length of this wire contains exactly 1.00 mol of silver? (density of Ag = 10.5 g/cm3)
A)52.3 m
B)222 m
C)13.1 m
D)2.01 m
E)890 m
A)52.3 m
B)222 m
C)13.1 m
D)2.01 m
E)890 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
22
How many atoms are in 4.39 g of CO2?
A)1.80 * 1023 atoms
B)6.01 * 1022 atoms
C)1.16 * 1026 atoms
D)6.04 * 1024 atoms
E)1.81 * 1025 atoms
A)1.80 * 1023 atoms
B)6.01 * 1022 atoms
C)1.16 * 1026 atoms
D)6.04 * 1024 atoms
E)1.81 * 1025 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the molar mass of nicotine, C10H14N2?
A)134 g/mol
B)148 g/mol
C)158 g/mol
D)210 g/mol
E)162 g/mol
A)134 g/mol
B)148 g/mol
C)158 g/mol
D)210 g/mol
E)162 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many moles of NH3 are there in 77.5 g of NH3?
A)0.220 mol
B)4.55 mol
C)14.0 mol
D)1.31 * 103 mol
E)None of the above.
A)0.220 mol
B)4.55 mol
C)14.0 mol
D)1.31 * 103 mol
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
25
Calculate the molecular mass of potassium permanganate, KMnO4.
A)52 amu
B)70 amu
C)110 amu
D)158 amu
E)176 amu
A)52 amu
B)70 amu
C)110 amu
D)158 amu
E)176 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
26
Formaldehyde has the formula CH2O. How many molecules are there in 0.11 g of formaldehyde?
A)6.1 * 10 -27
B)3.7 * 10 -3
C)4
D)2.2 * 1021
E)6.6 * 1022
A)6.1 * 10 -27
B)3.7 * 10 -3
C)4
D)2.2 * 1021
E)6.6 * 1022

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
27
A gold wire has a diameter of 1.00 mm. What length of this wire contains exactly 1.00 mol of gold? (density of Au = 17.0 g/cm3)
A)2630 m
B)3.69 m
C)251 m
D)14.8 m
E)62.7 m
A)2630 m
B)3.69 m
C)251 m
D)14.8 m
E)62.7 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the mass of 0.0250 mol of P2O5?
A)35.5 g
B)5676 g
C)0.0250 g
D)1.51 * 1022 g
E)3.55 g
A)35.5 g
B)5676 g
C)0.0250 g
D)1.51 * 1022 g
E)3.55 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
29
Calculate the mass of 3.00 moles of CF2Cl2.
A)3.00 g
B)174 g
C)363 g
D)1.81 * 1024 g
E)40.3 g
A)3.00 g
B)174 g
C)363 g
D)1.81 * 1024 g
E)40.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
30
How many moles of HCl are represented by 1.0 * 1019 HCl molecules?
A)1.7 * 10-5 mol
B)1.5 * 10-3 mol
C)1.0 * 1019 mol
D)36.5 mol
E)6.02 * 104 mol
A)1.7 * 10-5 mol
B)1.5 * 10-3 mol
C)1.0 * 1019 mol
D)36.5 mol
E)6.02 * 104 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
31
How many molecules are there in 8.0 g of ozone, O3?
A)3 molecules
B)3.6 * 1024 molecules
C)1.0 * 1023 molecules
D)3.0 * 1023 molecules
E)6.0 * 1023 molecules
A)3 molecules
B)3.6 * 1024 molecules
C)1.0 * 1023 molecules
D)3.0 * 1023 molecules
E)6.0 * 1023 molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
32
Calculate the molecular mass of menthol, C10H20O.
A)156 amu
B)140 amu
C)29 amu
D)146 amu
E)136 amu
A)156 amu
B)140 amu
C)29 amu
D)146 amu
E)136 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
33
The molecular formula of aspirin is C9H8O4. How many aspirin molecules are present in one 500-milligram tablet?
A)2.77 molecules
B)2.77 * 10-3 molecules
C)1.67 * 1024 molecules
D)1.67 * 1021 molecules
E)None of these is correct.
A)2.77 molecules
B)2.77 * 10-3 molecules
C)1.67 * 1024 molecules
D)1.67 * 1021 molecules
E)None of these is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
34
A copper wire has a diameter of 2.00 mm. What length of this wire contains exactly 1.00 mol of copper? (density of Cu = 8.92 g/cm3)
A)0.178 m
B)0.567 m
C)180 m
D)45.1 m
E)2.27 m
A)0.178 m
B)0.567 m
C)180 m
D)45.1 m
E)2.27 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many atoms are in 5.54 g of F2?
A)6.02 * 1023 atoms
B)0.146 atoms
C)0.292 atoms
D)8.78 * 1022 atoms
E)1.76 * 1023 atoms
A)6.02 * 1023 atoms
B)0.146 atoms
C)0.292 atoms
D)8.78 * 1022 atoms
E)1.76 * 1023 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
36
How many atoms are in 0.0728 g of PCl3?
A)1.28 * 1021 atoms
B)4.38 * 1022 atoms
C)4.39 * 1021 atoms
D)3.19 * 1020 atoms
E)6.02 * 1024 atoms
A)1.28 * 1021 atoms
B)4.38 * 1022 atoms
C)4.39 * 1021 atoms
D)3.19 * 1020 atoms
E)6.02 * 1024 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following samples contains the greatest number of atoms?
A)100 g of Pb
B)2.0 mole of Ar
C)0.1 mole of Fe
D)5 g of He
E)20 million O2 molecules
A)100 g of Pb
B)2.0 mole of Ar
C)0.1 mole of Fe
D)5 g of He
E)20 million O2 molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the molar mass of acetaminophen, C8H9NO2?
A)43 g/mol
B)76 g/mol
C)151 g/mol
D)162 g/mol
E)125 g/mol
A)43 g/mol
B)76 g/mol
C)151 g/mol
D)162 g/mol
E)125 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
39
Calculate the number of moles of cesium in 50.0 g of cesium.
A)0.376 mol
B)0.357 mol
C)2.66 mol
D)2.80 mol
E)0.0200 mol
A)0.376 mol
B)0.357 mol
C)2.66 mol
D)2.80 mol
E)0.0200 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
40
How many moles of CF4 are there in 171 g of CF4?
A)0.51 mol
B)1.94 mol
C)4.07 mol
D)88.0 mol
E)171 mol
A)0.51 mol
B)1.94 mol
C)4.07 mol
D)88.0 mol
E)171 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
41
How many sulfur atoms are present in 25.6 g of Al2(S2O3)3?
A)0.393
B)6
C)3.95 * 1022
D)7.90 * 1022
E)2.37 * 1023
A)0.393
B)6
C)3.95 * 1022
D)7.90 * 1022
E)2.37 * 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
42
Calculate the mass of 4.50 moles of chlorine gas, Cl2.
A)6.34 * 10 -2 g
B)4.5 g
C)15.7 g
D)160 g
E)319 g
A)6.34 * 10 -2 g
B)4.5 g
C)15.7 g
D)160 g
E)319 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
43
How many carbon atoms are there in 10 lbs of sugar, C12H22O11?
A)9.6 * 1025 atoms
B)8.0 * 1024 atoms
C)159 atoms
D)4.21 atoms
E)342 atoms
A)9.6 * 1025 atoms
B)8.0 * 1024 atoms
C)159 atoms
D)4.21 atoms
E)342 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the mass of 0.20 mole of C2H6O (ethanol)?
A)230 g
B)46 g
C)23 g
D)4.6 g
E)None of these.
A)230 g
B)46 g
C)23 g
D)4.6 g
E)None of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
45
How many grams of sulfur are there in 6.0 g of Fe2(SO4)3?
A)2.40 g
B)0.48 g
C)6.00 g
D)0.92 g
E)1.44 g
A)2.40 g
B)0.48 g
C)6.00 g
D)0.92 g
E)1.44 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
46
The empirical formula of a compound of uranium and fluorine that is composed of 67.6% uranium and 32.4% fluorine is
A)U2F.
B)U3F4.
C)UF4.
D)UF6.
E)UF8.
A)U2F.
B)U3F4.
C)UF4.
D)UF6.
E)UF8.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
47
The mass of four moles of molecular bromine (Br2)is
A)80 g.
B)320 g.
C)640 g.
D)140 g.
E)24 * 1023 g.
A)80 g.
B)320 g.
C)640 g.
D)140 g.
E)24 * 1023 g.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
48
How many grams of sodium are there in 10. g of sodium sulfate, Na2SO4?
A)0.16 g
B)0.32 g
C)3.2 g
D)1.6 g
E)142 g
A)0.16 g
B)0.32 g
C)3.2 g
D)1.6 g
E)142 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many grams of nitrogen are there in 7.5 g of Ca(NO3)2?
A)0.64 g
B)1.3 g
C)0.15 g
D)1.15 g
E)2.3 g
A)0.64 g
B)1.3 g
C)0.15 g
D)1.15 g
E)2.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
50
A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by
A)measuring the force of impact on a detecting screen, and then calculating the mass using force = mass * acceleration.
B)suspending the ions in an applied electric field, and then calculating mass by setting the downward gravitational force equal to the upward electrostatic force.
C)measuring the magnitude of deflection as the ions pass through a magnetic field to obtain the charge-to-mass ratio, and then calculating the mass from that ratio.
D)measuring the time it takes for the ions to hit the detector at a known distance to calculate the acceleration, and then calculating mass from force = mass * acceleration.
A)measuring the force of impact on a detecting screen, and then calculating the mass using force = mass * acceleration.
B)suspending the ions in an applied electric field, and then calculating mass by setting the downward gravitational force equal to the upward electrostatic force.
C)measuring the magnitude of deflection as the ions pass through a magnetic field to obtain the charge-to-mass ratio, and then calculating the mass from that ratio.
D)measuring the time it takes for the ions to hit the detector at a known distance to calculate the acceleration, and then calculating mass from force = mass * acceleration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
51
What is the mass of 8.25 * 1019 UF6 molecules?
A)352 g
B)0.0482 g
C)1.37 * 10-4 g
D)2.90 * 1022 g
E)8.25 * 1019 g
A)352 g
B)0.0482 g
C)1.37 * 10-4 g
D)2.90 * 1022 g
E)8.25 * 1019 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
52
How many moles of oxygen atoms are there in 10 moles of KClO3?
A)3 mol
B)3.3 mol
C)10 mol
D)30 mol
E)6.02 * 1024 mol
A)3 mol
B)3.3 mol
C)10 mol
D)30 mol
E)6.02 * 1024 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
53
How many O atoms are there in 51.4 g CaSO4?
A)4
B)2.40 * 1024
C)1.13
D)9.09 * 1023
E)2.28 * 1023
A)4
B)2.40 * 1024
C)1.13
D)9.09 * 1023
E)2.28 * 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
54
How many fluorine atoms are there in 65 g of CF4?
A)0.74 atoms
B)3.0 atoms
C)4.5 * 1023 atoms
D)1.8 * 1024 atoms
E)2.4 * 1023 atoms
A)0.74 atoms
B)3.0 atoms
C)4.5 * 1023 atoms
D)1.8 * 1024 atoms
E)2.4 * 1023 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
55
How many sulfur atoms are there in 21.0 g of Al2S3?
A)8.42 * 1022 atoms
B)2.53 * 1023 atoms
C)2.14 * 1023 atoms
D)6.02 * 1023 atoms
E)6.30 * 1026 atoms
A)8.42 * 1022 atoms
B)2.53 * 1023 atoms
C)2.14 * 1023 atoms
D)6.02 * 1023 atoms
E)6.30 * 1026 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the mass of 3.00 moles of ethanol, C2H6O?
A)4.99 * 10-24 g
B)138 g
C)6.52 * 10-2 g
D)50 g
E)1.81 * 1024 g
A)4.99 * 10-24 g
B)138 g
C)6.52 * 10-2 g
D)50 g
E)1.81 * 1024 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
57
How many moles of Cl atoms are there in 65.2 g CHCl3?
A)0.548 mol
B)1.09 mol
C)3.3 * 1023 mol
D)1.64 mol
E)3.0 mol
A)0.548 mol
B)1.09 mol
C)3.3 * 1023 mol
D)1.64 mol
E)3.0 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
58
How many moles of O atoms are in 25.7 g of CaSO4?
A)0.189 mol
B)0.755 mol
C)4.00 mol
D)1.14 * 1023 mol
E)4.55 * 1023 mol
A)0.189 mol
B)0.755 mol
C)4.00 mol
D)1.14 * 1023 mol
E)4.55 * 1023 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
59
How many sodium atoms are there in 6.0 g of Na3N?
A)3.6 * 1024 atoms
B)4.6 * 1022 atoms
C)1.3 * 1023 atoms
D)0.217 atoms
E)0.072 atoms
A)3.6 * 1024 atoms
B)4.6 * 1022 atoms
C)1.3 * 1023 atoms
D)0.217 atoms
E)0.072 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
60
The percent composition by mass of a compound is 76.0% C, 12.8% H, and 11.2% O. The molar mass of this compound is 284.5 g/mol. What is the molecular formula of the compound?
A)C10H6O
B)C9H18O
C)C16H28O4
D)C20H12O2
E)C18H36O2
A)C10H6O
B)C9H18O
C)C16H28O4
D)C20H12O2
E)C18H36O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which one of the following chemical reactions is balanced?
A)HCl + KMnO4 Cl2 + MnO2 + H2O + KCl
B)HCl + KMnO4 Cl2 + MnO2 + 2H2O + KCl
C)2HCl + 2KMnO4 Cl2 + MnO2 + 2H2O + 2KCl
D)6HCl + 2KMnO4 2Cl2 + 2MnO2 + 4H2O + 2KCl
E)8HCl + 2KMnO4 3Cl2 + 2MnO2 + 4H2O + 2KCl
A)HCl + KMnO4 Cl2 + MnO2 + H2O + KCl
B)HCl + KMnO4 Cl2 + MnO2 + 2H2O + KCl
C)2HCl + 2KMnO4 Cl2 + MnO2 + 2H2O + 2KCl
D)6HCl + 2KMnO4 2Cl2 + 2MnO2 + 4H2O + 2KCl
E)8HCl + 2KMnO4 3Cl2 + 2MnO2 + 4H2O + 2KCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
62
Balance the following equation using the smallest set of whole numbers, then add together the coefficients. Do not forget to count coefficients of one. The sum of the coefficients is __ SF4 + __ H2O __ H2SO3 + __ HF
A)4.
B)6.
C)7.
D)9.
E)none of these.
A)4.
B)6.
C)7.
D)9.
E)none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
63
When 22.0 g NaCl and 21.0 g H2SO4 are mixed and react according to the equation below, which is the limiting reagent?
2NaCl + H2SO4 Na2SO4 + 2HCl
A)NaCl
B)H2SO4
C)Na2SO4
D)HCl
E)No reagent is limiting.
2NaCl + H2SO4 Na2SO4 + 2HCl
A)NaCl
B)H2SO4
C)Na2SO4
D)HCl
E)No reagent is limiting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
64
The percent composition by mass of an unknown chlorinated hydrocarbon was found to be 37.83% C, 6.35% H, and 55.83% Cl by mass. What is the empirical formula of this compound?
A)C2H4Cl
B)C3H7Cl
C)C3H6Cl2
D)C4H9Cl
E)C5H11Cl
A)C2H4Cl
B)C3H7Cl
C)C3H6Cl2
D)C4H9Cl
E)C5H11Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
65
When a chemical equation is balanced, it will have a set of whole number coefficients that cannot be reduced to smaller whole numbers. What is the coefficient for O2 when the following combustion reaction of a hydrocarbon is balanced? ___ C7H14 + ___ O2 ___ CO2 + ___ H2O
A)42
B)21
C)11
D)10
E)none of these
A)42
B)21
C)11
D)10
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the coefficient preceding O2 when the following combustion reaction of a fatty acid is properly balanced using the smallest set of whole numbers? __ C18H36O2 + __ O2 __ CO2 + __ H2O
A)1
B)8
C)9
D)26
E)27
A)1
B)8
C)9
D)26
E)27

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
67
Balance the following equation using the smallest set of whole numbers, then add together the coefficients. Do not forget to count coefficients of one. The sum of the coefficients is ___ Al + ___ H2SO4 ___ Al2(SO4)3 + ___ H2
A)3.
B)5.
C)6.
D)9.
E)12.
A)3.
B)5.
C)6.
D)9.
E)12.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
68
Vanadium(V)oxide reacts with calcium according to the chemical equation below. When 10.0 moles of V2O5 are mixed with 10.0 moles of Ca, which is the limiting reagent? V2O5(s)+ 5Ca(l) 2V(l)+ 5CaO(s)
A)V2O5
B)Ca
C)V
D)CaO
E)No reagent is limiting.
A)V2O5
B)Ca
C)V
D)CaO
E)No reagent is limiting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
69
Ammonia reacts with diatomic oxygen to form nitric oxide and water vapor: 4NH3 + 5O2 4NO + 6H2O
When 40.0 g NH3 and 50.0 g O2 are allowed to react, which is the limiting reagent?
A)NH3
B)O2
C)NO
D)H2O
E)No reagent is limiting.
When 40.0 g NH3 and 50.0 g O2 are allowed to react, which is the limiting reagent?
A)NH3
B)O2
C)NO
D)H2O
E)No reagent is limiting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the coefficient of H2O when the following equation is properly balanced with smallest set of whole numbers?
___ Al4C3 + ___ H2O ___ Al(OH)3 + ___ CH4
A)3
B)4
C)6
D)12
E)24
___ Al4C3 + ___ H2O ___ Al(OH)3 + ___ CH4
A)3
B)4
C)6
D)12
E)24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
71
When balanced with smallest set of whole numbers, the coefficient of O2 in the following equation is __ C2H4 + __ O2 __ CO2 + __ H2O
A)1.
B)2.
C)3.
D)4.
E)6.
A)1.
B)2.
C)3.
D)4.
E)6.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
72
Balance the equation below using the smallest set of whole numbers. What is the coefficient of H2O?
___ PCl3(l)+ ___ H2O(l) ___ H3PO3(aq) + ___ HCl(aq)
A)1
B)2
C)3
D)5
E)none of these
___ PCl3(l)+ ___ H2O(l) ___ H3PO3(aq) + ___ HCl(aq)
A)1
B)2
C)3
D)5
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
73
An organic thiol compound is 38.66% C, 9.73% H, and 51.61% S by mass. What is the empirical formula of this compound?
A)C2H6S
B)C3H8S
C)C4H10S
D)C4H12S
E)C5H14S
A)C2H6S
B)C3H8S
C)C4H10S
D)C4H12S
E)C5H14S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
74
Ammonia reacts with diatomic oxygen to form nitric oxide and water vapor: 4NH3 + 5O2 4NO + 6H2O
When 20.0 g NH3 and 50.0 g O2 are allowed to react, which is the limiting reagent?
A)NH3
B)O2
C)NO
D)H2O
E)No reagent is limiting.
When 20.0 g NH3 and 50.0 g O2 are allowed to react, which is the limiting reagent?
A)NH3
B)O2
C)NO
D)H2O
E)No reagent is limiting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
75
A compound was discovered whose composition by mass is 85.6% C and 14.4% H. Which of the following could be the molecular formula of this compound?
A)CH4
B)C2H4
C)C3H4
D)C2H6
E)C3H8
A)CH4
B)C2H4
C)C3H4
D)C2H6
E)C3H8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
76
What is the coefficient of H2SO4 when the following equation is properly balanced with the smallest set of whole numbers?
___ Ca3(PO4)2 + ___ H2SO4 ___ CaSO4 + ___ H3PO4
A)3
B)8
C)10
D)11
E)none of these
___ Ca3(PO4)2 + ___ H2SO4 ___ CaSO4 + ___ H3PO4
A)3
B)8
C)10
D)11
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
77
What is the coefficient of O2 when the following equation is properly balanced with the smallest set of whole numbers?
___ CH3OH + ___ O2 ___ CO2 + ___ H2O
A)1
B)2
C)3
D)7
E)none of these
___ CH3OH + ___ O2 ___ CO2 + ___ H2O
A)1
B)2
C)3
D)7
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
78
Balance the following equation using the smallest set of whole numbers, then add together the coefficients. Don't forget to count coefficients of one. The sum of the coefficients is ___ Cr + ___ H2SO4 ___ Cr2(SO4)3 + ___ H2
A)4.
B)9.
C)11.
D)13.
E)15.
A)4.
B)9.
C)11.
D)13.
E)15.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the coefficient of H2O when the following equation is properly balanced with the smallest set of whole numbers?
___ Na + ___ H2O ___ NaOH + ___ H2
A)1
B)2
C)3
D)4
E)5
___ Na + ___ H2O ___ NaOH + ___ H2
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck
80
Balance the following equation using the smallest set of whole numbers, then add together the coefficients. Do not forget to count coefficients of one. The sum of the coefficients is ___ CH4 + ___ Cl2 ___ CCl4 + ___ HCl
A)4.
B)6.
C)8.
D)10.
E)12.
A)4.
B)6.
C)8.
D)10.
E)12.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 168 في هذه المجموعة.
فتح الحزمة
k this deck








