Deck 8: Electron Configurations and Periodicity
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/100
العب
ملء الشاشة (f)
Deck 8: Electron Configurations and Periodicity
1
What is the maximum number of electrons that can be accommodated in the n = 1 shell?
A)2
B)6
C)4
D)8
E)10
A)2
B)6
C)4
D)8
E)10
2
2
Which of the following statements is true concerning the electron configuration [Ne]3p2?
A)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Pauli exclusion principle.
B)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates Hund's rule.
C)This configuration is the ground-state electron configuration for a Mg atom.
D)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Heisenberg uncertainty principle.
E)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Aufbau principle.
A)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Pauli exclusion principle.
B)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates Hund's rule.
C)This configuration is the ground-state electron configuration for a Mg atom.
D)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Heisenberg uncertainty principle.
E)This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Aufbau principle.
This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Aufbau principle.
3
Which of the following electron configurations corresponds to the ground state of an atom of a transition element?
A)1s22s22p5
B)1s22s22p63s23p63d104s24p2
C)1s22s22p63s23p63d74s2
D)1s22s22p63s23p64s2
E)1s22s22p63s23p1
A)1s22s22p5
B)1s22s22p63s23p63d104s24p2
C)1s22s22p63s23p63d74s2
D)1s22s22p63s23p64s2
E)1s22s22p63s23p1
1s22s22p63s23p63d74s2
4
What is the maximum number of electrons that can occupy one p orbital?
A)14
B)2
C)10
D)1
E)6
A)14
B)2
C)10
D)1
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following statements is incorrect?
A)Stern and Gerlach discovered electron spin by passing silver atoms through a magnetic field.
B)Hund's rule states that electrons are placed in the orbitals of a subshell in such a way as to give a maximum number of unpaired electrons.
C)The Pauli exclusion principle states that each electron in an atom must have its own unique set of quantum numbers.
D)Valence electrons consist of those electrons not contained within a noble-gas core or a pseudo-noble-gas core.
E)The building-up principle states that electrons are added to atoms in order of increasing principal quantum number.
A)Stern and Gerlach discovered electron spin by passing silver atoms through a magnetic field.
B)Hund's rule states that electrons are placed in the orbitals of a subshell in such a way as to give a maximum number of unpaired electrons.
C)The Pauli exclusion principle states that each electron in an atom must have its own unique set of quantum numbers.
D)Valence electrons consist of those electrons not contained within a noble-gas core or a pseudo-noble-gas core.
E)The building-up principle states that electrons are added to atoms in order of increasing principal quantum number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
6
The maximum number of electrons that can be accommodated in an s subshell is
A)6.
B)14.
C)10.
D)1.
E)2.
A)6.
B)14.
C)10.
D)1.
E)2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
7
According to the building-up principle or aufbau principle,which subshell is typically filled next after the 5f subshell?
A)6d
B)4p
C)7s
D)3d
E)5s
A)6d
B)4p
C)7s
D)3d
E)5s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
8
The Pauli exclusion principle states that
A)the wavelength of a photon of light times its frequency is equal to the speed of light.
B)no two electrons in the same atom can have the same set of four quantum numbers.
C)both the position of an electron and its momentum cannot be known simultaneously very accurately.
D)the wavelength and mass of a subatomic particle are related by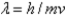 .
.
E)an electron can have either particle character or wave character.
A)the wavelength of a photon of light times its frequency is equal to the speed of light.
B)no two electrons in the same atom can have the same set of four quantum numbers.
C)both the position of an electron and its momentum cannot be known simultaneously very accurately.
D)the wavelength and mass of a subatomic particle are related by
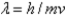 .
.E)an electron can have either particle character or wave character.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following orbital occupancy designations is incorrect?
A)3d4
B)4d1
C)2p4
D)1s1
E)1s3
A)3d4
B)4d1
C)2p4
D)1s1
E)1s3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which element is found in the s-block of the periodic table?
A)Ra
B)Ar
C)Db
D)Np
E)none of the above
A)Ra
B)Ar
C)Db
D)Np
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
11
The ground-state valence-shell configuration of a particular atom is 4f145d56s2 .The element to which this atom belongs is a
A)noble gas.
B)s-block main-group element.
C)p-block main-group element.
D)transition element.
E)inner transition element.
A)noble gas.
B)s-block main-group element.
C)p-block main-group element.
D)transition element.
E)inner transition element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following electron configurations is impossible,according to the Pauli exclusion principle?
A)1s22s22p5
B)1s22s22p3
C)1s22s5
D)1s22s22p63s1
E)1s22s22p2
A)1s22s22p5
B)1s22s22p3
C)1s22s5
D)1s22s22p63s1
E)1s22s22p2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
13
The ground-state valence-shell configuration of a particular atom is 4d105s25p1.The element to which this atom belongs is a
A)noble gas.
B)inner transition element.
C)p-block main-group element.
D)transition element.
E)s-block main-group element.
A)noble gas.
B)inner transition element.
C)p-block main-group element.
D)transition element.
E)s-block main-group element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
14
Two elements that have the same ground-state valence shell configuration of ns2np2 are
A)K and Mg.
B)O and Se.
C)Al and Ga.
D)Ge and Pb.
E)Mg and Ca.
A)K and Mg.
B)O and Se.
C)Al and Ga.
D)Ge and Pb.
E)Mg and Ca.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the maximum number of electrons in an atom that have the set of quantum numbers n= 2 and l= 1?
A)10
B)2
C)14
D)18
E)6
A)10
B)2
C)14
D)18
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which principle or rule is violated by the following orbital diagram of an atom in its ground state?
1s 2s 2p




A)Pauli exclusion principle
B)Hund's rule
C)Heisenberg uncertainty principle
D)No rules or principles are violated by this orbital diagram.
E)Building-up principle
1s 2s 2p





A)Pauli exclusion principle
B)Hund's rule
C)Heisenberg uncertainty principle
D)No rules or principles are violated by this orbital diagram.
E)Building-up principle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following orbital diagrams violates the Pauli exclusion principle?
1s 2s 2p
A)




B)




C)




D)




E)




1s 2s 2p
A)





B)





C)





D)





E)






فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following electron configurations represents an excited state of the indicated atom?
A)Ne: 1s2 2s2 2p6
B)N: 1s2 2s2 2p3
C)P: 1s2 2s2 2p6 3s2 3p2 4s1
D)Na: 1s2 2s2 2p6 3s2 3p2 3s1
E)He: 1s2
A)Ne: 1s2 2s2 2p6
B)N: 1s2 2s2 2p3
C)P: 1s2 2s2 2p6 3s2 3p2 4s1
D)Na: 1s2 2s2 2p6 3s2 3p2 3s1
E)He: 1s2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which principle or rule is violated by the following orbital diagram of an atom in its ground state?
1s 2s 2p




A)Pauli exclusion principle
B)Aufbau principle
C)No rules or principles are violated by this orbital diagram.
D)Heisenberg uncertainty principle
E)Hund's rule
1s 2s 2p





A)Pauli exclusion principle
B)Aufbau principle
C)No rules or principles are violated by this orbital diagram.
D)Heisenberg uncertainty principle
E)Hund's rule

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following have one or more filled d subshells in their ground state electron configuration?
A)Br
B)H
C)Ti
D)Cr
E)P
A)Br
B)H
C)Ti
D)Cr
E)P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the total number of electrons in p orbitals in a ground-state iron atom?
A)6
B)18
C)12
D)24
E)30
A)6
B)18
C)12
D)24
E)30

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the ground-state electron configuration of tantalum (Ta)?
A)1s22s22p63s23p64s24p64d104f145s25p3
B)1s22s22p63s23p63d104s24p64d105s25p3
C)1s22s22p63s23p63d104s24p64d104f145s25p65d36s2
D)1s22s22p63s23p63d104s24p64d105s25p65d3
E)1s22s22p63s23p63d104s24p64d104f3
A)1s22s22p63s23p64s24p64d104f145s25p3
B)1s22s22p63s23p63d104s24p64d105s25p3
C)1s22s22p63s23p63d104s24p64d104f145s25p65d36s2
D)1s22s22p63s23p63d104s24p64d105s25p65d3
E)1s22s22p63s23p63d104s24p64d104f3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the valence-shell electron configuration for the fourth-period element in Group VA?
A)4s25p5
B)5s25p5
C)4s24p3
D)5s24p5
E)4s23d3
A)4s25p5
B)5s25p5
C)4s24p3
D)5s24p5
E)4s23d3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
24
An element that has the same ground state valence-shell electron configuration as sodium is
A)lithium.
B)barium.
C)boron.
D)lead.
E)xenon.
A)lithium.
B)barium.
C)boron.
D)lead.
E)xenon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the ground-state electron configuration of sulfur (S)?
A)[Ne]3s33p3
B)[Ar]3s23p4
C)[Ar]3p6
D)[Ne]3s23p4
E)[Ne]3p6
A)[Ne]3s33p3
B)[Ar]3s23p4
C)[Ar]3p6
D)[Ne]3s23p4
E)[Ne]3p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
26
How many valence electrons does an arsenic atom have?
A)5
B)6
C)7
D)2
E)33
A)5
B)6
C)7
D)2
E)33

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
27
The quantum numbers of an atom's highest-energy valence electrons are n = 5 and l = 1.The element to which this atom belongs could be a
A)inner transition metal.
B)alkali metal.
C)s-block main-group element.
D)transition metal.
E)p-block main-group element.
A)inner transition metal.
B)alkali metal.
C)s-block main-group element.
D)transition metal.
E)p-block main-group element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
28
The ground-state valence-shell configuration of a particular atom is 4s24p3.This valence-shell electron configuration identifies the atom as
A)a p-block main-group element.
B)a noble gas.
C)a transition element.
D)an inner transition element.
E)an s-block main-group element.
A)a p-block main-group element.
B)a noble gas.
C)a transition element.
D)an inner transition element.
E)an s-block main-group element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following statements is incorrect?
A)A p-block main-group element belonging to period n has a completely filled (n - 1)d subshell.
B)All noble gases have completely filled shells.
C)All s-block main-group elements have only one or two valence electrons.
D)Carbon and silicon have the same number of valence electrons.
E)All elements in the n = 4 period have a partially or completely filled n = 4 shell.
A)A p-block main-group element belonging to period n has a completely filled (n - 1)d subshell.
B)All noble gases have completely filled shells.
C)All s-block main-group elements have only one or two valence electrons.
D)Carbon and silicon have the same number of valence electrons.
E)All elements in the n = 4 period have a partially or completely filled n = 4 shell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
30
All of the following ground-state electron configurations are correct except
A)Fe:[Ar]4s24d6.
B)Ca:[Ar]4s2.
C)In:[Kr]4d105s25p1.
D)Cu:[Ar]3d104s1.
E)Xe:[Kr]4d105s25p6.
A)Fe:[Ar]4s24d6.
B)Ca:[Ar]4s2.
C)In:[Kr]4d105s25p1.
D)Cu:[Ar]3d104s1.
E)Xe:[Kr]4d105s25p6.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following statements is true concerning the electron configuration [Ne]3s13p1?
A)It may represent a ground-state electron configuration of a Al+ cation.
B)It may represent an excited-state electron configuration of a Mg atom.
C)It may represent an excited-state electron configuration of a Ne- anion.
D)It may represent a ground-state electron configuration of a Mg+ cation.
E)It may represent a ground-state electron configuration of a Na+ cation.
A)It may represent a ground-state electron configuration of a Al+ cation.
B)It may represent an excited-state electron configuration of a Mg atom.
C)It may represent an excited-state electron configuration of a Ne- anion.
D)It may represent a ground-state electron configuration of a Mg+ cation.
E)It may represent a ground-state electron configuration of a Na+ cation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
32
The angular momentum quantum number of the two highest-energy valence electrons in an atom of lead is
A)4.
B)0.
C)1.
D)2.
E)3.
A)4.
B)0.
C)1.
D)2.
E)3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
33
The elements that are filling the 5f subshell are called
A)alkali metals.
B)transition elements.
C)lanthanides.
D)actinides.
E)main-group elements.
A)alkali metals.
B)transition elements.
C)lanthanides.
D)actinides.
E)main-group elements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
34
What noble gas core would be used when writing the ground state electron configuration for magnesium (Mg)?
A)[Ne]
B)[Xe]
C)[Rn]
D)[He]
E)[Ar]
A)[Ne]
B)[Xe]
C)[Rn]
D)[He]
E)[Ar]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following sets of four quantum numbers (n,l,ml, ms)correctly describes one of the valence electrons in a ground-state radium atom?
A)7 1 0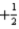
B)6 1 0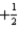
C)7 2 0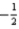
D)7 2 0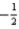
E)7 0 0 +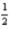
A)7 1 0
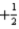
B)6 1 0
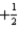
C)7 2 0
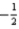
D)7 2 0
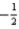
E)7 0 0 +
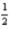

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
36
If the electron could have a third spin state (that is, 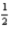 ,-
,- 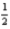
,and 0),what would be the ground-state electron configuration of carbon?
A)1s22s4
B)1s22s32p1
C)1s32s3
D)1s22s22p2
E)1s32s22p1
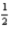 ,-
,- 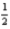
,and 0),what would be the ground-state electron configuration of carbon?
A)1s22s4
B)1s22s32p1
C)1s32s3
D)1s22s22p2
E)1s32s22p1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the ground-state electron configuration of terbium (Tb)?
A)1s22s22p63s23p63d104s24p64d105s25p65d96s2
B)1s22s22p63s23p63d104s24p64d104f145s25p3
C)1s22s22p63s23p63d104s24p64d105s25p65d106s1
D)1s22s22p63s23p63d104s24p64d94f105s25p66s2
E)1s22s22p63s23p63d104s24p64d104f95s25p66s2
A)1s22s22p63s23p63d104s24p64d105s25p65d96s2
B)1s22s22p63s23p63d104s24p64d104f145s25p3
C)1s22s22p63s23p63d104s24p64d105s25p65d106s1
D)1s22s22p63s23p63d104s24p64d94f105s25p66s2
E)1s22s22p63s23p63d104s24p64d104f95s25p66s2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following may represent an excited-state electron configuration for a cobalt atom?
A)[Ar]3d54s1
B)[Ar]3d64s2
C)[Ar]3d84s1
D)[Ar]3d64s1
E)[Ar]3d74s2
A)[Ar]3d54s1
B)[Ar]3d64s2
C)[Ar]3d84s1
D)[Ar]3d64s1
E)[Ar]3d74s2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which ground-state electron configuration is incorrect?
A)Br:[Ar]3d104s24p5
B)K:[Ar]4s1
C)Fe:[Ar]3d5
D)Na:1s22s22p63s1
E)Zn:[Ar]3d104s2
A)Br:[Ar]3d104s24p5
B)K:[Ar]4s1
C)Fe:[Ar]3d5
D)Na:1s22s22p63s1
E)Zn:[Ar]3d104s2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following sets of four quantum numbers (n,l,ml, ms)correctly describes an electron occupying a d orbital of an element in the third row of the transition metals?
A)4 2 -1 -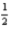
B)5 2 2 +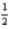
C)5 3 -1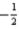
D)4 1 0 -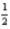
E)5 0 0 +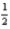
A)4 2 -1 -
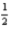
B)5 2 2 +
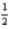
C)5 3 -1
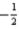
D)4 1 0 -
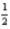
E)5 0 0 +

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following statements concerning the periodic table is incorrect?
A)The elements in a given group or family have similar chemical properties.
B)The chemical characteristics of the elements are periodic functions of their atomic numbers.
C)All the elements are arranged in order of increasing atomic weight.
D)Mendeleev left spaces in his periodic table for undiscovered elements.
E)Mendeleev received most of the credit for the early development of the periodic table.
A)The elements in a given group or family have similar chemical properties.
B)The chemical characteristics of the elements are periodic functions of their atomic numbers.
C)All the elements are arranged in order of increasing atomic weight.
D)Mendeleev left spaces in his periodic table for undiscovered elements.
E)Mendeleev received most of the credit for the early development of the periodic table.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
42
The statement that "the lowest-energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals" is known as
A)the aufbau principle.
B)Hund's rule.
C)the Pauli exclusion principle.
D)Heisenberg uncertainty principle.
E)the quantum model.
A)the aufbau principle.
B)Hund's rule.
C)the Pauli exclusion principle.
D)Heisenberg uncertainty principle.
E)the quantum model.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
43
Who was the first chemist to recognize patterns in chemical properties of the elements?
A)Bohr
B)Dobereiner
C)Meyer
D)Mendeleev
E)Newlands
A)Bohr
B)Dobereiner
C)Meyer
D)Mendeleev
E)Newlands

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
44
In general,atomic radii
A)decrease from left to right in a period and increase down a group.
B)increase from left to right in a period and decrease down a group.
C)do not change across a period or a group.
D)decrease from left to right and decrease down a group.
E)increase from left to right in a period and increase down a group.
A)decrease from left to right in a period and increase down a group.
B)increase from left to right in a period and decrease down a group.
C)do not change across a period or a group.
D)decrease from left to right and decrease down a group.
E)increase from left to right in a period and increase down a group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
45
How many unpaired electrons are found in the ground state electron configuration of silicon (Si)?
A)2
B)3
C)5
D)0
E)1
A)2
B)3
C)5
D)0
E)1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
46
The element whose atoms in the ground state have two half-filled orbitals is
A)K.
B)Be.
C)Tl.
D)O.
E)As.
A)K.
B)Be.
C)Tl.
D)O.
E)As.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the following atoms is paramagnetic in its ground state?
A)sodium (Na)
B)nobelium (No)
C)neon (Ne)
D)mercury (Hg)
E)barium (ba)
A)sodium (Na)
B)nobelium (No)
C)neon (Ne)
D)mercury (Hg)
E)barium (ba)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following species would be expected to have chemical properties most similar to those of the nitrogen atom?
A)nitride ion
B)nitrite ion
C)phosphate ion
D)phosphide ion
E)phosphorus atom
A)nitride ion
B)nitrite ion
C)phosphate ion
D)phosphide ion
E)phosphorus atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following orbital diagrams represents a diamagnetic atom?
1s 2s 2p
A)
B)
C)
D)
E)
1s 2s 2p
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following statements is true?
A)The krypton 1s orbital is smaller than the helium 1s orbital because krypton's nuclear charge draws the electrons closer.
B)The krypton 1s orbital is smaller than the helium 1s orbital because krypton's p and d orbitals crowd the s orbitals.
C)The krypton 1s orbital and the helium 1s orbital are the same size because both s orbitals can have only two electrons.
D)The krypton 1s orbital is larger than the helium 1s orbital because krypton contains more electrons.
E)The krypton 1s orbital is larger than the helium 1s orbital because krypton's ionization energy is lower so it's easier to remove electrons.
A)The krypton 1s orbital is smaller than the helium 1s orbital because krypton's nuclear charge draws the electrons closer.
B)The krypton 1s orbital is smaller than the helium 1s orbital because krypton's p and d orbitals crowd the s orbitals.
C)The krypton 1s orbital and the helium 1s orbital are the same size because both s orbitals can have only two electrons.
D)The krypton 1s orbital is larger than the helium 1s orbital because krypton contains more electrons.
E)The krypton 1s orbital is larger than the helium 1s orbital because krypton's ionization energy is lower so it's easier to remove electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
51
An atom of which of the following elements has the smallest atomic radius?
A)P
B)Cl
C)S
D)Na
E)Si
A)P
B)Cl
C)S
D)Na
E)Si

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which principle or rule is violated by the following orbital diagram of an atom in its ground state?
1s 2s 2p




A)Heisenberg uncertainty principle
B)No rules or principles are violated by this orbital diagram.
C)aufbau principle
D)Hund's rule
E)Pauli exclusion principle
1s 2s 2p





A)Heisenberg uncertainty principle
B)No rules or principles are violated by this orbital diagram.
C)aufbau principle
D)Hund's rule
E)Pauli exclusion principle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
53
An atom of which of the following elements is not diamagnetic in the ground state?
A)Sr
B)Kr
C)All are diamagnetic.
D)Cd
E)Pt
A)Sr
B)Kr
C)All are diamagnetic.
D)Cd
E)Pt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
54
The ground-state electron configuration of a Mn2+ ion is 1s22s22p63s23p63d5.Therefore,Mn2+ is
A)paramagnetic with five unpaired electrons.
B)diamagnetic.
C)paramagnetic with one unpaired electron.
D)paramagnetic with three unpaired electrons.
E)paramagnetic with two unpaired electrons.
A)paramagnetic with five unpaired electrons.
B)diamagnetic.
C)paramagnetic with one unpaired electron.
D)paramagnetic with three unpaired electrons.
E)paramagnetic with two unpaired electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
55
When arranged in order of increasing atomic number,the elements exhibit periodicity for all the following properties except
A)electron affinity.
B)color.
C)ionization energy.
D)electron configuration.
E)atomic radius.
A)electron affinity.
B)color.
C)ionization energy.
D)electron configuration.
E)atomic radius.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following elements would be expected to have chemical and physical properties most similar to those of the krypton (Kr)?
A)neon (Ne)
B)gallium (Ga)
C)sodium (Na)
D)tin (Sn)
E)chlorine (Cl)
A)neon (Ne)
B)gallium (Ga)
C)sodium (Na)
D)tin (Sn)
E)chlorine (Cl)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following orbital diagrams represent(s)a paramagnetic atom?
1s 2s 3s
1)
2)
3)
A)3 only
B)2 and 3
C)1 only
D)1 and 2
E)2 only
1s 2s 3s
1)

2)

3)

A)3 only
B)2 and 3
C)1 only
D)1 and 2
E)2 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
58
A section of the periodic table with all identification features removed is shown below.
V
W
X
Y
Z
Which element has the smallest atomic radius?
A)W
B)Y
C)X
D)Z
E)V
V
W
X
Y
Z
Which element has the smallest atomic radius?
A)W
B)Y
C)X
D)Z
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following orbital diagrams represents a paramagnetic atom?
1s 2s 2p
A)




B)




C)




D)




E)




1s 2s 2p
A)





B)





C)





D)





E)






فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
60
Fe has ____ that is(are)unpaired in its d orbitals.
A)2 electrons
B)3 electrons
C)1 electron
D)4 electrons
E)none of these
A)2 electrons
B)3 electrons
C)1 electron
D)4 electrons
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
61
An atom of which of the following elements has the largest atomic radius?
A)Ca
B)Sr
C)Mg
D)Ba
E)Be
A)Ca
B)Sr
C)Mg
D)Ba
E)Be

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
62
The change in energy for which of the following processes corresponds to the second ionization energy of magnesium?
A)Mg(g)→ Mg2+(g)+ 2e-
B)Mg(g)→ Mg+(g)+ e-
C)Mg+(g)→ Mg2+(g)+ e-
D)Mg-(g)→ Mg(g)+ e-
E)Mg(g)+ e- → Mg-(g)
A)Mg(g)→ Mg2+(g)+ 2e-
B)Mg(g)→ Mg+(g)+ e-
C)Mg+(g)→ Mg2+(g)+ e-
D)Mg-(g)→ Mg(g)+ e-
E)Mg(g)+ e- → Mg-(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
63
An atom of which of the following elements has the smallest atomic radius?
A)Tl
B)B
C)In
D)Ga
E)Al
A)Tl
B)B
C)In
D)Ga
E)Al

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
64
Below are data on the first four ionization energies for a fictitious element X.
First ionization energy = 500 kJ/mol
Second ionization energy = 2000 kJ/mol
Third ionization energy = 3500 kJ/mol
Fourth ionization energy = 25000 kJ/mol
From the data,which of the following statements is incorrect?
A)The third ionization energy is greater than the second ionization energy because X2+ has a bigger charge than X+.
B)X could belong to Group IIIA.
C)X could belong to Group IIIB.
D)X could belong to group VA.
E)The fourth ionization energy is much greater than the third ionization energy because X3+ consists of a noble-gas core or a pseudo-noble-gas core.
First ionization energy = 500 kJ/mol
Second ionization energy = 2000 kJ/mol
Third ionization energy = 3500 kJ/mol
Fourth ionization energy = 25000 kJ/mol
From the data,which of the following statements is incorrect?
A)The third ionization energy is greater than the second ionization energy because X2+ has a bigger charge than X+.
B)X could belong to Group IIIA.
C)X could belong to Group IIIB.
D)X could belong to group VA.
E)The fourth ionization energy is much greater than the third ionization energy because X3+ consists of a noble-gas core or a pseudo-noble-gas core.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
65
Rank the following atoms in order of the largest to smallest atomic radius: B,B,B,C.
A)C > B > B > B
B)B > C > B > B
C)B > B > C > B
D)B > B > B > C
A)C > B > B > B
B)B > C > B > B
C)B > B > C > B
D)B > B > B > C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
66
An atom of which of the following elements has the smallest ionization energy?
A)Cl
B)P
C)Si
D)Na
E)S
A)Cl
B)P
C)Si
D)Na
E)S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
67
An atom of which of the following elements has the largest ionization energy?
A)Po
B)Pb
C)Bi
D)At
E)Cs
A)Po
B)Pb
C)Bi
D)At
E)Cs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
68
An atom of which of the following elements has the smallest first ionization energy?
A)Rb
B)Si
C)F
D)P
E)Ca
A)Rb
B)Si
C)F
D)P
E)Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
69
An atom of which of the following elements has the highest fourth ionization energy?
A)Al
B)Se
C)Si
D)Ga
E)As
A)Al
B)Se
C)Si
D)Ga
E)As

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
70
An atom of which of the following elements has the largest atomic radius?
A)Ge
B)Rb
C)F
D)Ca
E)P
A)Ge
B)Rb
C)F
D)Ca
E)P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
71
The change in energy for which of the following processes represents the first ionization energy of iodine?
A)I(g)→ I2+(g)+ 2e-
B)I(g)+ e - → I-(g)
C)2I-(g)→ I2(s)+ 2e-
D)I(g)→ I+(g)+ e-
E)I-(g)→ I+(g)+ 2e-
A)I(g)→ I2+(g)+ 2e-
B)I(g)+ e - → I-(g)
C)2I-(g)→ I2(s)+ 2e-
D)I(g)→ I+(g)+ e-
E)I-(g)→ I+(g)+ 2e-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
72
The statement that the first ionization energy for an oxygen atom is lower than the first ionization energy for a nitrogen atom is
A)inconsistent with the general trend relating changes in ionization energy across a period from left to right and due to the fact that oxygen has one doubly occupied 2p orbital and nitrogen does not.
B)consistent with the general trend relating changes in ionization energy across a period from left to right because it is harder to take an electron from an oxygen atom than from a nitrogen atom.
C)consistent with the general trend relating changes in ionization energy across a period from left to right because it is easier to take an electron from an oxygen atom than from a nitrogen atom.
D)incorrect.
E)inconsistent with the general trend relating changes in ionization energy across a period from left to right and due to the fact that the oxygen atom has two doubly occupied 2p orbitals and nitrogen has only one.
A)inconsistent with the general trend relating changes in ionization energy across a period from left to right and due to the fact that oxygen has one doubly occupied 2p orbital and nitrogen does not.
B)consistent with the general trend relating changes in ionization energy across a period from left to right because it is harder to take an electron from an oxygen atom than from a nitrogen atom.
C)consistent with the general trend relating changes in ionization energy across a period from left to right because it is easier to take an electron from an oxygen atom than from a nitrogen atom.
D)incorrect.
E)inconsistent with the general trend relating changes in ionization energy across a period from left to right and due to the fact that the oxygen atom has two doubly occupied 2p orbitals and nitrogen has only one.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
73
An atom of which of the following elements has the largest first ionization energy?
A)B
B)In
C)Ga
D)Al
E)Tl
A)B
B)In
C)Ga
D)Al
E)Tl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
74
An atom of which of the following elements has the smallest first ionization energy?
A)Te
B)Po
C)Se
D)O
E)S
A)Te
B)Po
C)Se
D)O
E)S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
75
The change in energy for which of the following processes corresponds to the first ionization energy of beryllium?
A)Be(g)→ Be+(g)+ e-
B)Be(s)→ Be+(s)+ e-
C)Be(s)→ Be+(g)+ e-
D)Be(g)→ Be2+(g)+ 2e-
E)Be(s)+ e- → Be-(s)
A)Be(g)→ Be+(g)+ e-
B)Be(s)→ Be+(s)+ e-
C)Be(s)→ Be+(g)+ e-
D)Be(g)→ Be2+(g)+ 2e-
E)Be(s)+ e- → Be-(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following properties,in general,increases from left to right across a period in the periodic table?
A)ionic charge
B)atomic radius
C)density
D)ionization energy
E)metallic character
A)ionic charge
B)atomic radius
C)density
D)ionization energy
E)metallic character

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
77
An atom of which of the following elements has the smallest atomic radius?
A)Cl
B)Rb
C)Mg
D)Si
E)As
A)Cl
B)Rb
C)Mg
D)Si
E)As

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
78
In which of the series of elements listed below would the elements have most nearly the same atomic radius?
A)Na,K,Rb,Cs
B)F,Cl,Br,I
C)Na,Mg,Al,Si
D)Sc,Ti,V,Cr
E)B,Si,As,Te
A)Na,K,Rb,Cs
B)F,Cl,Br,I
C)Na,Mg,Al,Si
D)Sc,Ti,V,Cr
E)B,Si,As,Te

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
79
An atom of which of the following elements has the largest first ionization energy?
A)Ca
B)K
C)Ge
D)F
E)P
A)Ca
B)K
C)Ge
D)F
E)P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
80
An atom of which of the following elements has the largest atomic radius?
A)F
B)N
C)Li
D)O
E)C
A)F
B)N
C)Li
D)O
E)C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck








