Deck 16: Electrical Energy and Capacitance: Part A
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/33
العب
ملء الشاشة (f)
Deck 16: Electrical Energy and Capacitance: Part A
1
A heat engine exhausts 4000 J of heat while performing 2000 J of useful work.What is the efficiency of the engine?
A) 15%
B) 33%
C) 40%
D) 60%
A) 15%
B) 33%
C) 40%
D) 60%
33%
2
A closed 2.0-L container holds 3.0 mol of an ideal gas.If 150 J of heat is added,what is the change in internal energy of the system?
A) zero
B) 100 J
C) 150 J
D) 200 J
A) zero
B) 100 J
C) 150 J
D) 200 J
150 J
3
A heat engine receives 6000 J of heat from its combustion process and loses 3600 J through the exhaust and friction.What is its efficiency?
A) 33%
B) 40%
C) 67%
D) 73%
A) 33%
B) 40%
C) 67%
D) 73%
40%
4
In an isobaric process 3.6 × 104 J of work is done on a quantity of gas while its volume changes from 2.3 m3 to 1.1 m3.What is the pressure during this process?
A) 1.2 × 104 Pa
B) 2.4 × 104 Pa
C) 3.0 × 104 Pa
D) 8.3 × 104 Pa
A) 1.2 × 104 Pa
B) 2.4 × 104 Pa
C) 3.0 × 104 Pa
D) 8.3 × 104 Pa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
5
A cylinder containing an ideal gas has a volume of 3.0 m3 and a pressure of 1.0 × 105 Pa at a temperature of 300 K.The cylinder is placed against a metal block that is maintained at 900 K,and the gas expands as the pressure remains constant until the temperature of the gas reaches 900 K.The change in internal energy of the gas is +6.0 × 105 J.How much heat did the gas absorb?
A) 0
B) 6.0 × 105 J
C) 12 × 105 J
D) 9.0 × 105 J
A) 0
B) 6.0 × 105 J
C) 12 × 105 J
D) 9.0 × 105 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
6
Consider a Carnot cycle starting at the state of highest pressure and smallest volume.Call the isothermal expansion from this state step 1,the adiabatic expansion which follows step 2,the isothermal compression that then follows step 3,and the adiabatic compression which returns the system to the initial state step 4.In which steps does the internal energy of the system increase?
A) 1 and 2
B) 3 and 4
C) 1 and 3
D) 2 and 4
A) 1 and 2
B) 3 and 4
C) 1 and 3
D) 2 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
7
An electrical generating plant operates at a boiler temperature of 220°C and exhausts the unused heat into a nearby river at 19°C.If the generating plant has a power output of 800 megawatts (MW)and if the actual efficiency is 3/5 the theoretical efficiency,how much heat per second must be delivered to the boiler? (0°C = 273 K)
A) 5200 MW
B) 1810 MW
C) 3270 MW
D) 2620 MW
A) 5200 MW
B) 1810 MW
C) 3270 MW
D) 2620 MW

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
8
A 4-mol ideal gas system undergoes an adiabatic process where it expands and does 28 J of work on its environment.What is its change in internal energy?
A) 28 J
B) −28 J
C) zero
D) −7 J
A) 28 J
B) −28 J
C) zero
D) −7 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
9
Three Carnot engines operate between temperature reservoirs as follows: Engine A: Th = 1300 K,Tc = 1000 K;Engine B: Th = 1000 K,Tc = 700 K;Engine C: Th = 500 K,Tc = 350 K.Which two engines have the same thermal efficiency?
A) A and B
B) B and C
C) A and C
D) No two have the same thermal efficiency.
A) A and B
B) B and C
C) A and C
D) No two have the same thermal efficiency.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
10
An electrical generating plant operates at a boiler temperature of 200°C and exhausts the unused heat into a nearby river at 20°C.What is the maximum theoretical efficiency of the plant? (0°C = 273 K)
A) 61%
B) 38%
C) 21%
D) 41%
A) 61%
B) 38%
C) 21%
D) 41%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
11
A heat engine operating between a pair of hot and cold reservoirs with respective temperatures of 500 K and 300 K will have what maximum efficiency?
A) 60%
B) 50%
C) 40%
D) 30%
A) 60%
B) 50%
C) 40%
D) 30%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
12
A turbine takes in 1000 K steam and exhausts the steam at a temperature of 400 K.What is the maximum theoretical efficiency of this system?
A) 24%
B) 40%
C) 50%
D) 60%
A) 24%
B) 40%
C) 50%
D) 60%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
13
A 2.0-mol ideal gas system is maintained at a constant volume of 4.0 L.If 200 J of heat is added,what is the work done on the system?
A) zero
B) 5.0 J
C) -6.7 J
D) 20 J
A) zero
B) 5.0 J
C) -6.7 J
D) 20 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the processes on a monatomic ideal gas given below will result in the temperature of the gas increasing? W is the work done by the gas.
A) a process where Q = W
B) a process where Q = -2W
C) a process where Q = 2W
D) None of the above will increase the temperature of the gas.
A) a process where Q = W
B) a process where Q = -2W
C) a process where Q = 2W
D) None of the above will increase the temperature of the gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
15
The volume of an ideal gas changes from 0.40 to 0.55 m3 although its pressure remains constant at 50,000 Pa.What work is done by the system ON its environment?
A) -7500 J
B) −200,000 J
C) 7500 J
D) 200,000 J
A) -7500 J
B) −200,000 J
C) 7500 J
D) 200,000 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
16
A system is acted on by its surroundings in such a way that it receives 50 J of heat while simultaneously doing 70 J of work.What is its net change in internal energy?
A) 70 J
B) −20 J
C) zero
D) 20 J
A) 70 J
B) −20 J
C) zero
D) 20 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
17
A 4-mol ideal gas system undergoes an adiabatic process where it expands and does 28 J of work on its environment.How much heat is received by the system?
A) −20 J
B) zero
C) +28 J
D) +7 J
A) −20 J
B) zero
C) +28 J
D) +7 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
18
A quantity of monatomic ideal gas expands adiabatically from a volume of 2.0 liters to 4.0 liters.If the initial pressure is P0,what is the final pressure?
A) 9.0P0
B) 6.2P0
C) 0.31P0
D) 0.16P0
A) 9.0P0
B) 6.2P0
C) 0.31P0
D) 0.16P0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
19
A system has an increase in internal energy of 60 J while 90 J was removed from the system by heat.How much work was involved during this process and was it done on the system or done on its environment?
A) 30 J on the system
B) 30 J on the environment
C) 150 J on the system
D) 150 J on the environment
A) 30 J on the system
B) 30 J on the environment
C) 150 J on the system
D) 150 J on the environment

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
20
An electrical power plant manages to send 88% of the heat produced in the burning of fossil fuel into the water-to-steam conversion.Of the heat carried by the steam,30% is converted to the mechanical energy of the spinning turbine.Which of the following choices best describes the overall efficiency of the heat-to-work conversion in the plant (as a percentage)?
A) greater than 88%
B) 64%
C) 30%
D) less than 30%
A) greater than 88%
B) 64%
C) 30%
D) less than 30%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
21
The surface of the Sun is at approximately 5700 K,and the temperature of the Earth's surface is about 290 K.What total entropy change occurs when 2000 J of heat energy is transferred from the Sun to the Earth?
A) 5.78 J/K
B) 5.97 J/K
C) 7.24 J/K
D) 6.55 J/K
A) 5.78 J/K
B) 5.97 J/K
C) 7.24 J/K
D) 6.55 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
22
You have a PV diagram for a device going through a cyclic process forming a closed loop on the diagram.How can you tell from the diagram whether the device is acting as a heat engine and not as a refrigerator?
A) The loop is longer than it is high which means it is acting as a refrigerator.
B) The loop is higher than it is long which means it is acting as a refrigerator.
C) The loop proceeds clockwise which means it is acting as a heat engine.
D) The loop proceeds counterclockwise which means it is acting as a heat engine.
A) The loop is longer than it is high which means it is acting as a refrigerator.
B) The loop is higher than it is long which means it is acting as a refrigerator.
C) The loop proceeds clockwise which means it is acting as a heat engine.
D) The loop proceeds counterclockwise which means it is acting as a heat engine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
23
A gasoline engine with an efficiency of 40.0% operates between a high temperature T1 and a low temperature T2 = 320 K.If this engine operates with Carnot efficiency,what is the high-side temperature T1?
A) 1070 K
B) 868 K
C) 533 K
D) 457 K
A) 1070 K
B) 868 K
C) 533 K
D) 457 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
24
The laws of thermodynamics can be related to the following: (i)the measurement of temperature, (ii)the lowest temperature possible, (iii)conservation of energy,and (iv)only part of heat energy available may be turned into work.Which of these involve the zeroth and third law?
A) (i)and (ii)
B) (ii)and (iv)
C) (i)and (iii)
D) (iii)and (iv)
A) (i)and (ii)
B) (ii)and (iv)
C) (i)and (iii)
D) (iii)and (iv)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
25
Consider a Carnot cycle starting at the state of highest pressure and smallest volume.Call the isothermal expansion that starts at this state step 1,the adiabatic expansion which follows step 2,the isothermal compression that then follows step 3,and the adiabatic compression which returns the system to the initial state step 4.In which step does the entropy of the system increase?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
26
An avalanche of ice and snow of mass 2500 kg slides a vertical distance of 160 m down a mountainside.If the temperature of the ice,snow,mountain,and surrounding air are all at 0°C,what is the change in entropy of the universe?
A) 14,000 J/K
B) 10,000 J/K
C) 1400 J/K
D) 1100 J/K
A) 14,000 J/K
B) 10,000 J/K
C) 1400 J/K
D) 1100 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
27
In an isothermal process where the pressure decreases from 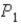
To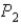
,does the internal energy of the system increase,decrease,or stay the same;and does the entropy of the system increase or decrease?
A) The internal energy decreases and the entropy increases.
B) The internal energy stays the same and the entropy increases.
C) The internal energy decreases and the entropy decreases.
D) The internal energy stays the same and the entropy decreases.
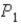
To
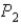
,does the internal energy of the system increase,decrease,or stay the same;and does the entropy of the system increase or decrease?
A) The internal energy decreases and the entropy increases.
B) The internal energy stays the same and the entropy increases.
C) The internal energy decreases and the entropy decreases.
D) The internal energy stays the same and the entropy decreases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
28
During each cycle of operation a refrigerator absorbs 45 cal from the freezer compartment and expels 85 cal to the room.If one cycle occurs every 8.0 s,how many minutes will it take to freeze 500 g of water,initially at 0°C? (Lv = 80 cal/g)
A) 800 min
B) 4400 min
C) 120 min
D) 150 min
A) 800 min
B) 4400 min
C) 120 min
D) 150 min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
29
A cylinder containing an ideal gas has a volume of 3.0 m3 and a pressure of 1.0 × 105 Pa at a temperature of 300 K.The cylinder is placed against a metal block that is maintained at 900 K and the gas expands as the pressure remains constant until the temperature of the gas reaches 900 K.The change in internal energy of the gas is 9.0 × 105 J.Find the change in entropy of the block associated with the heat transfer to the gas.
A) 0
B) +670 J/K
C) −1700 J/K
D) −1100 J/K
A) 0
B) +670 J/K
C) −1700 J/K
D) −1100 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
30
The laws of thermodynamics can be related to the following: (i)the measurement of temperature, (ii)the lowest temperature possible, (iii)conservation of energy,and (iv)only part of heat energy available may be turned into work.Which of these involve the first and second law?
A) (i)and (ii)
B) (ii)and (iv)
C) (i)and (iii)
D) (iii)and (iv)
A) (i)and (ii)
B) (ii)and (iv)
C) (i)and (iii)
D) (iii)and (iv)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
31
When gasoline is burned,it gives off 46,000 J/g of heat energy.If an automobile uses 13.0 kg of gasoline per hour with an efficiency of 18%,what is the average horsepower output of the engine? (1 hp = 746 W)
A) 47 hp
B) 110 hp
C) 67 hp
D) 40 hp
A) 47 hp
B) 110 hp
C) 67 hp
D) 40 hp

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
32
A 600-MW electric power plant has an efficiency of 30%.It loses its waste heat in large cooling towers.Approximately how much waste heat (in MJ)is discharged to the atmosphere per second?
A) 1400 MJ
B) 1900 MJ
C) 800 MJ
D) 560 MJ
A) 1400 MJ
B) 1900 MJ
C) 800 MJ
D) 560 MJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
33
Two kilograms of water at 1.00 atm at the boiling point of 100°C is heated until all the water vaporizes.What is its change in entropy? (For water,Lv = 2.26 × 106 J/kg)
A) 12,100 J/K
B) 6060 J/K
C) 3030 J/K
D) 1220 J/K
A) 12,100 J/K
B) 6060 J/K
C) 3030 J/K
D) 1220 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck








