Deck 5: Solution Concentration
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/76
العب
ملء الشاشة (f)
Deck 5: Solution Concentration
1
The conversion factor for converting from moles of HPO42-- to equivalents is: 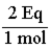
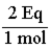
True
2
When you have your blood drawn, the most common method of expressing the plasma level of sodium and potassium is as mEq/L.
True
3
Dialysis and osmosis are used for the same purposes.
False
4
Solution contains 55 mg of magnesium in 2.5 L of solution. The concentration of this solution is 2.2 mg/dL.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
5
There is a 9 M aqueous HCl solution in the stock room, but a 5 M solution is required for an experiment. Doubling the volume of the 9 M sample with water will produce the 5 M solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
6
Consider the following graph. 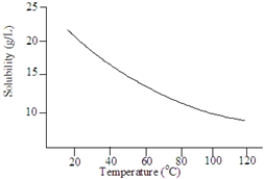 The solute is probably a gas.
The solute is probably a gas.
 The solute is probably a gas.
The solute is probably a gas.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
7
Solution concentration is an intensive property.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
8
The concentration of cholesterol in plasma was determined to be 215 mg/dL. The mass of cholesterol in 59.1 mL of this plasma is 127 mg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
9
Putting a celery stick in distilled water results in the uptake of water by the celery.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
10
In cases of cerebral endema, a hypertonic solution is administered. The goal of giving the patient the hypertonic solution would be to pull fluid from the cells through the process of osmosis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
11
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  water will have moved from the right-hand container to the left hand container..
water will have moved from the right-hand container to the left hand container..
 water will have moved from the right-hand container to the left hand container..
water will have moved from the right-hand container to the left hand container..
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
12
The total molarity of an intravenous solution is given on the label as 151 mEq/L. The osmolarity of this solution is also 151 mEq/L.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
13
The following equation can be used when C represents either a M or % (w/v) concentration. 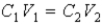
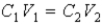

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
14
For a 2.00 M solution, the conversion factor for determining the number of moles of solute in a given volume of solution is: 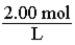
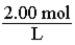

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
15
A normal saline solution is a 0.90% (w/v) aqueous solution of NaCl. This is the same as: 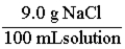
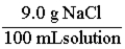

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
16
The %(v/v) of a solution can be defined as: 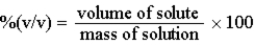
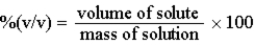

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
17
The solubility of Na2SO4 will probably increase with increasing temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
18
If you have 9 g of NaCl in 1 L of water, this solution is 0.9% NaCl and is called normal saline or NS.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
19
The solubility of a compound in water was measured and found to be 0.7 g/L. This compound would be classified as insoluble.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
20
The osmotic pressure of a 0.10 M NaCl solution will be the same as that of a 0.10 M urea solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the mass of salt in a 400.0 gram-sample of salt water which is 1.50% (w/w) salt?
A)1.50 g
B)2.67 g
C)3.00 g
D)6.00 g
A)1.50 g
B)2.67 g
C)3.00 g
D)6.00 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
22
A solution is made by dissolving 22.5 mL of oil in enough gasoline to give 60.5 mL of solution. What is the % (v/v) of oil in the solution?
A)37.2 % (v/v)
B)269% (v/v)
C)0.372% (v/v)
D)27.1% (v/v)
A)37.2 % (v/v)
B)269% (v/v)
C)0.372% (v/v)
D)27.1% (v/v)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
23
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution?
A)0.100 M
B)1.00 M
C)0.0250 M
D)0.400 M
A)0.100 M
B)1.00 M
C)0.0250 M
D)0.400 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the molar mass of ibuprofen, C13H18O2?
A)29.0 g/mol
B)206.3 g/mol
C)289.4 g/mol
D)377.7 g/mol
A)29.0 g/mol
B)206.3 g/mol
C)289.4 g/mol
D)377.7 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
25
How many grams of solid KCl are needed to prepare 250.0 mL of 0.235 M solution?
A)9.32 g
B)31.3 g
C)15.6 g
D)4.38 g
A)9.32 g
B)31.3 g
C)15.6 g
D)4.38 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
26
A solution contains 35.5 mg of Vitamin C in 175 mL of solution. The concentration of this solution could be expressed as:
A)203 ppm.
B)0.0203% (m/v).
C)20.3 mg/dL.
D)All of the above could be used.
A)203 ppm.
B)0.0203% (m/v).
C)20.3 mg/dL.
D)All of the above could be used.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the molarity of a solution containing 0.585 mol of lactic acid in 250.0 mL of solution?
A)146 M
B)2.34 M
C)0.427 M
D)0.146 M
A)146 M
B)2.34 M
C)0.427 M
D)0.146 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
28
A solution has a concentration of 16 ppm. Which of the following is another way to describe the concentration of this solution?
A)0.016 ppb
B)0.16 ppb
C)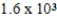 ppb
ppb
D)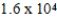 ppb
ppb
A)0.016 ppb
B)0.16 ppb
C)
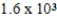 ppb
ppbD)
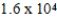 ppb
ppb
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the total molarity of solute particles in a 0.250 M solution of (NH4)2SO4?
A)0.250 M
B)0.500 M
C)0.750 M
D)0.0833 M
A)0.250 M
B)0.500 M
C)0.750 M
D)0.0833 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
30
How would the following solution be classified? 0.05 M in KCl and 0.14 M in glucose
A)isotonic
B)hypertonic
C)hypotonic
A)isotonic
B)hypertonic
C)hypotonic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
31
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  there will be no change in the liquid levels.
there will be no change in the liquid levels.
 there will be no change in the liquid levels.
there will be no change in the liquid levels.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following is associated with cells in a hypertonic solution?
A)crenation
B)hemolysis
C)reverse osmosis
D)none of these
A)crenation
B)hemolysis
C)reverse osmosis
D)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
33
Calculate the total molarity of solute particles in a solution that contains 0.050 M glucose (a nonelectrolyte) and 0.200 M CaCl2.
A)0.250 M
B)0.450 M
C)0.650 M
D)0.350 M
A)0.250 M
B)0.450 M
C)0.650 M
D)0.350 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
34
The following list gives the concentration of the major components of blood plasma.
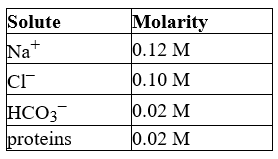
What is the total concentration of all the minor components in the plasma?
A)0.02 M
B)0.28 M
C)0.12 M
D)Cannot be determined with the given information.

What is the total concentration of all the minor components in the plasma?
A)0.02 M
B)0.28 M
C)0.12 M
D)Cannot be determined with the given information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
35
If 1.00 mol of each of the following solutes is dissolved in 2.00 L of an aqueous solution, which solution contains the largest number of solute particles?
A)LiBr
B)sucrose
C)Ca(NO3)2
D)Na3PO4
A)LiBr
B)sucrose
C)Ca(NO3)2
D)Na3PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
36
Calculate the number of moles of ZnCl2, in 180.0 mL of 0.330 M solution.
A)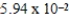 mol
mol
B)1.83 mol
C)0.545 mol
D)59.4 mol
A)
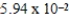 mol
molB)1.83 mol
C)0.545 mol
D)59.4 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
37
Lactated Ringer's solution 109 mEq/L of Cl-. What is the mass of the chloride ion in 225 mL of this solution?
A)24.5 g
B)0.0245 g
C)0.869 g
D)0.109 g
A)24.5 g
B)0.0245 g
C)0.869 g
D)0.109 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
38
What will happen to a red blood cell if placed in the following solution? 0.14 M lactose (a nonelectrolyte)
A)crenation
B)hemolysis
C)nothing
A)crenation
B)hemolysis
C)nothing

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following pass through both osmotic and dialysis membranes?
A)solvent molecules
B)large molecules
C)small molecules that are larger than solvent molecules
D)both solvent and small molecules
A)solvent molecules
B)large molecules
C)small molecules that are larger than solvent molecules
D)both solvent and small molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
40
Saline solutions (NaCl in water) used to deliver intravenous drugs are 0.89%(w/v). What mass of NaCl would be needed to prepare 450.0 mL of such a solution?
A)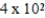 g
g
B)0.89 g
C)4.0 g
D)5.1 g
A)
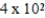 g
gB)0.89 g
C)4.0 g
D)5.1 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
41
A normal concentration of sodium (Na) in blood plasma is 141 mEq/L (US average range is 135-145 mEq/L). How many mg of sodium are there in a 10.0 mL sample of his blood plasma?
A)141 mg
B)14.1 mg
C)1.41 mg
D)1.40 × 103 mg
A)141 mg
B)14.1 mg
C)1.41 mg
D)1.40 × 103 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
42
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 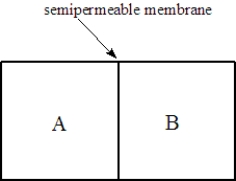
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.

Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
43
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 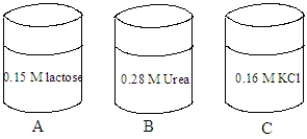
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
The tonicity of the solution in container C is ______________________.

Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
The tonicity of the solution in container C is ______________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
44
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.

Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
45
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container C. The cell will undergo ____________________.

Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container C. The cell will undergo ____________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
46
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container B. The cell will undergo ____________________.

Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container B. The cell will undergo ____________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
47
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container A. The cell will undergo _____________________.

Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container A. The cell will undergo _____________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
48
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.

Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
49
A stockroom attendant has a 15.0% (w/v) solution of KOH. What volume of this solution should she use if she needs to prepare 20.0 mL of a 10.0% (w/v) solution?
A)6.67 mL
B)7.50 mL
C)13.3 mL
D)15.0 mL
A)6.67 mL
B)7.50 mL
C)13.3 mL
D)15.0 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
50
How many mL of 6.00 M HCl are needed to prepare 1.50 L of 0.200 M HCl solution?
A)1.80 × 104 mL
B)125 mL
C)2.00 × 10−3 mL
D)50.0 mL
A)1.80 × 104 mL
B)125 mL
C)2.00 × 10−3 mL
D)50.0 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
51
An oral rehydration solution contains 30 mEq/L of citrate3-, what is the molarity of citrate in this solution?
A)0.09 M
B)90 M
C)10 M.
D)0.01 M
A)0.09 M
B)90 M
C)10 M.
D)0.01 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
52
Based on the following graph, approximately what minimum temperature would be needed to dissolved 20 g of this solute in 1.00 L of water? 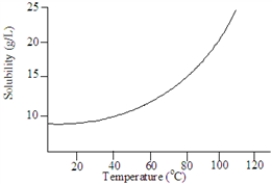
A)9C
B)100C
C)35C
D)80C

A)9C
B)100C
C)35C
D)80C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
53
If a solution contains 3.25 mol of Al3+, how many equivalents of Al3+ are present?
A)3.25 Eq
B)1.08 Eq
C)6.50 Eq
D)9.75 Eq
A)3.25 Eq
B)1.08 Eq
C)6.50 Eq
D)9.75 Eq

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
54
Based on the following graph, the solubility of this substance at 80 °C is approximately: 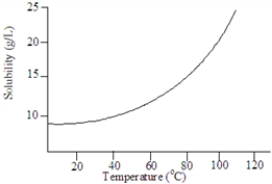
A)25 g/L.
B)15 g/L.
C)10 g/L
D)20 g/L

A)25 g/L.
B)15 g/L.
C)10 g/L
D)20 g/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
55
A student is preparing a sugar water solution to make rock candy. When the student adds sugar to the solution, the added sugar does not dissolve. Which kind of solution does the student have?
A)a saturated solution
B)a solution at its solubility limit
C)an unsaturated solution
D)a suspension
A)a saturated solution
B)a solution at its solubility limit
C)an unsaturated solution
D)a suspension

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
56
If the seminpermeable membrane has pores too small to allow glucose to pass through, ______________will not occur.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
57
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.010 M glucose, and solution B is 0.050 M glucose. The glucose will dialyze to the _____________________.

Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.010 M glucose, and solution B is 0.050 M glucose. The glucose will dialyze to the _____________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
58
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.

Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
59
A solution contains 5.75 mg of magnesium ions in 332 mL of solution. What is the concentration of the solution in mEq/L?
A)0.712 mEq/L
B)0.473 mEq/L
C)1.42 mEq/L.
D)34.6 mEq/L
A)0.712 mEq/L
B)0.473 mEq/L
C)1.42 mEq/L.
D)34.6 mEq/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
60
A solution is prepared by adding 25.0 mL of 1.30 M glucose solution to a flask, and then adding enough water to give a final volume of 200.0 mL. What is the molarity of the solution?
A)0.260 M
B)0.163 M
C)6.50 M
D)1.24 M
A)0.260 M
B)0.163 M
C)6.50 M
D)1.24 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
61
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 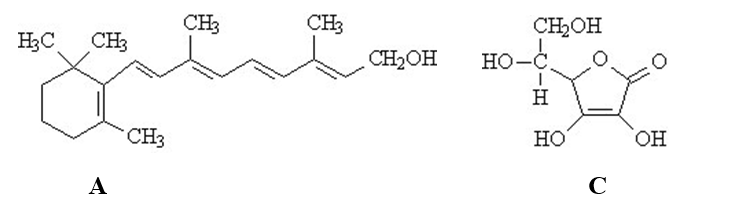
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The vitamin that would not be stored by the human body is ______.

Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The vitamin that would not be stored by the human body is ______.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
62
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.
Upon standing the water molecules will move in which direction?
 Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.Upon standing the water molecules will move in which direction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
63
For the following questions,fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
As the size of the hydrophobic portion of a molecule increases, the solubility in water will ___________.
increase
decrease
remains constant
cannot predict
As the size of the hydrophobic portion of a molecule increases, the solubility in water will ___________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
64
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.
Upon standing the sucrose molecules will move in which direction?
 Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.Upon standing the sucrose molecules will move in which direction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
65
For the following questions,fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
When a beverage can is opened the pressure of the gas above the liquid will___________ causing the solubility of the gas to ____________.
increase
decrease
remains constant
cannot predict
When a beverage can is opened the pressure of the gas above the liquid will___________ causing the solubility of the gas to ____________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
66
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.
Calculate the parts per million (ppm) of a solution prepared by dissolving 7.45 mg of glucose in enough water to produce 145.0 mL of solution?
 Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.Calculate the parts per million (ppm) of a solution prepared by dissolving 7.45 mg of glucose in enough water to produce 145.0 mL of solution?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
67
Consider the following structure. 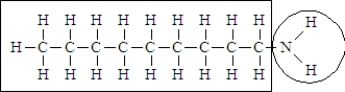
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
The portion of the molecule in the box is classified as ______________________ and that in the circle is classified as_____________________.

Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
The portion of the molecule in the box is classified as ______________________ and that in the circle is classified as_____________________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
68
Consider the following structure. 
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
This molecule is probably____________________in water.

Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
This molecule is probably____________________in water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
69
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense. The vitamin that would be the most soluble in the upper layer is ______.
The vitamin that would be the most soluble in the upper layer is ______.

Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense.
 The vitamin that would be the most soluble in the upper layer is ______.
The vitamin that would be the most soluble in the upper layer is ______.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
70
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.
What is the total molarity of a solution containing 25.7 g of sucrose (C12H22O11) and 16.1 g of ribose (C5H10O5) dissolved in enough water to produce 850.0 mL of solution?
 Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.What is the total molarity of a solution containing 25.7 g of sucrose (C12H22O11) and 16.1 g of ribose (C5H10O5) dissolved in enough water to produce 850.0 mL of solution?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
71
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.
Calculate the percent concentration (m/v) of a solution prepared by dissolving 6.45 g of glucose in enough water to produce 85.0 mL of solution?
 Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.Calculate the percent concentration (m/v) of a solution prepared by dissolving 6.45 g of glucose in enough water to produce 85.0 mL of solution?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
72
Write the conversion factor that corresponds to 7.75%(w/v) NaCl.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
73
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The vitamin that would be the most hydrophobic is ______.

Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The vitamin that would be the most hydrophobic is ______.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
74
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.
Upon standing the glucose molecules will move in which direction?
 Answer the following questions as appropriate with: right,left,or no movement.
Answer the following questions as appropriate with: right,left,or no movement.Upon standing the glucose molecules will move in which direction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
75
For the following questions,fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
For many ionic solids such as NaHCO3 water solubility will ___________ at higher temperatures..
increase
decrease
remains constant
cannot predict
For many ionic solids such as NaHCO3 water solubility will ___________ at higher temperatures..

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck
76
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The vitamin that would be classified as fat-soluble is ______.

Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
The vitamin that would be classified as fat-soluble is ______.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 76 في هذه المجموعة.
فتح الحزمة
k this deck








