Deck 12: Organohalides: Nucleophilic Substitutions and Eliminations
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/37
العب
ملء الشاشة (f)
Deck 12: Organohalides: Nucleophilic Substitutions and Eliminations
1
Instructions: Circle the correct response in each set below.
Refer to instructions.The least reactive compound in an SN1 reaction.
Refer to instructions.The least reactive compound in an SN1 reaction.

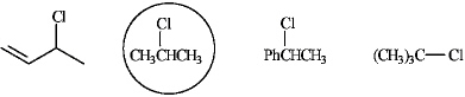
2
Instructions: Consider the pair of reactions below to answer the following question(s). 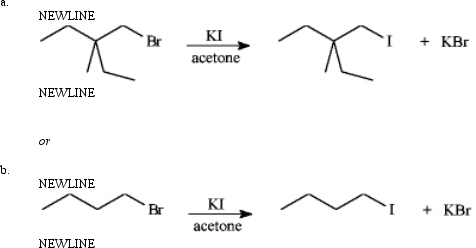
Refer to instructions.The mechanism for these reactions is:
A)SN2
B)E2
C)SN1
D)E1

Refer to instructions.The mechanism for these reactions is:
A)SN2
B)E2
C)SN1
D)E1
SN2
3
Complete the following reaction sequence by drawing the structure of the products in the boxes provided.

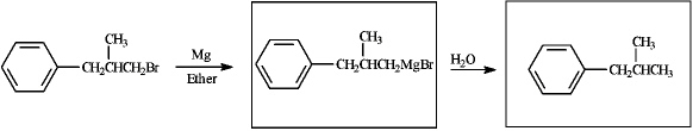
4
If the pKa of methane is 60 and that of ethene is 44,predict if the following reaction occurs and if so write the formula for the product.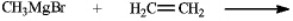 The reaction
The reaction
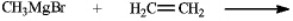 The reaction
The reaction 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
5
Instructions: Consider the reaction below to answer the following question(s).
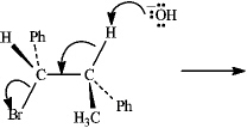
Refer to instructions.Draw a Newman projection of the reactive conformation of the starting material.

Refer to instructions.Draw a Newman projection of the reactive conformation of the starting material.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
6
Instructions: Consider the pair of reactions below to answer the following question(s). 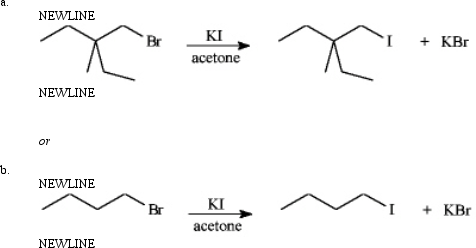
-Refer to instructions.The alkyl bromide starting materials in these reactions are classified as:
A)3
B)2
C)1
D)4

-Refer to instructions.The alkyl bromide starting materials in these reactions are classified as:
A)3
B)2
C)1
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
7
Instructions: Circle the correct response in each set below.
Refer to instructions.The best leaving group in an elimination reaction.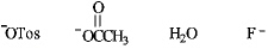
Refer to instructions.The best leaving group in an elimination reaction.
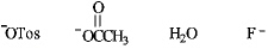

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
8
Instructions: Circle the correct response in each set below.
Refer to instructions.The least reactive compound in an SN2 reaction.Explain your choice.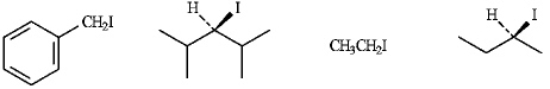
Refer to instructions.The least reactive compound in an SN2 reaction.Explain your choice.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
9
Instructions: Consider the pair of reactions below to answer the following question(s). ![<strong>Instructions: Consider the pair of reactions below to answer the following question(s). Refer to instructions.Which of the following statements is false?</strong> A)The kinetics of these reactions are second-order B)The kinetics of these reactions are first-order in the nucleophile C)The rate law would be of the form R = k[alkyl halide]<sup>2</sup> D)The kinetics of these reactions are first-order in alkyl halide](https://d2lvgg3v3hfg70.cloudfront.net/TB4938/11ea7ca1_4b48_0007_9d3f_5195c895d56a_TB4938_00.jpg)
Refer to instructions.Which of the following statements is false?
A)The kinetics of these reactions are second-order
B)The kinetics of these reactions are first-order in the nucleophile
C)The rate law would be of the form R = k[alkyl halide]2
D)The kinetics of these reactions are first-order in alkyl halide
![<strong>Instructions: Consider the pair of reactions below to answer the following question(s). Refer to instructions.Which of the following statements is false?</strong> A)The kinetics of these reactions are second-order B)The kinetics of these reactions are first-order in the nucleophile C)The rate law would be of the form R = k[alkyl halide]<sup>2</sup> D)The kinetics of these reactions are first-order in alkyl halide](https://d2lvgg3v3hfg70.cloudfront.net/TB4938/11ea7ca1_4b48_0007_9d3f_5195c895d56a_TB4938_00.jpg)
Refer to instructions.Which of the following statements is false?
A)The kinetics of these reactions are second-order
B)The kinetics of these reactions are first-order in the nucleophile
C)The rate law would be of the form R = k[alkyl halide]2
D)The kinetics of these reactions are first-order in alkyl halide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
10
Instructions: Consider the reaction below to answer the following question(s). 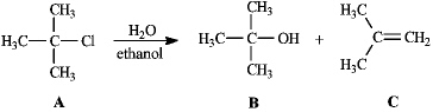
Refer to instructions.Compound C is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product

Refer to instructions.Compound C is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
11
Instructions: Consider the reaction below to answer the following question(s).
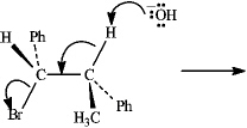
Refer to instructions.Write the product that results from the indicated electron flow in the reaction,showing any resulting stereochemistry

Refer to instructions.Write the product that results from the indicated electron flow in the reaction,showing any resulting stereochemistry

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
12
Instructions: Consider the reaction below to answer the following question(s). 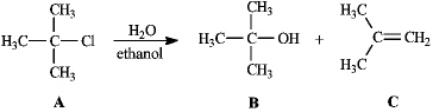
Refer to instructions.Compound B is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product

Refer to instructions.Compound B is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
13
Instructions: Consider the reaction below to answer the following question. 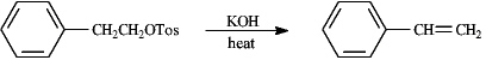
Refer to instructions.The mechanism for this reaction is:
A)SN2
B)E2
C)SN1
D)E1

Refer to instructions.The mechanism for this reaction is:
A)SN2
B)E2
C)SN1
D)E1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
14
Instructions: Consider the pair of reactions below to answer the following question(s). 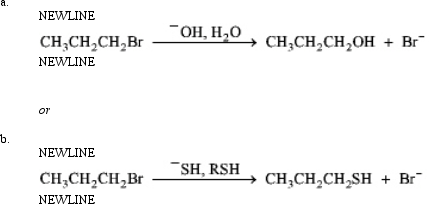
Refer to instructions.If the alkyl halide in each of these reactions was an alkyl chloride instead of the bromide,
A)the reaction rate would decrease.
B)a better leaving group would be involved.
C)a polar aprotic solvent would be needed.
D)DG‡ would be decreased.

Refer to instructions.If the alkyl halide in each of these reactions was an alkyl chloride instead of the bromide,
A)the reaction rate would decrease.
B)a better leaving group would be involved.
C)a polar aprotic solvent would be needed.
D)DG‡ would be decreased.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
15
Instructions: Consider the pair of reactions below to answer the following question(s).
a.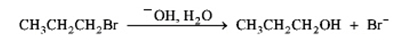
or
b.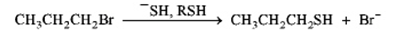
Consider the reactions above.
a) Which reaction would be predicted to be faster?
b) Classify the reactions as SN1 or SN2?
c) Explain your answers to the questions above.
a.
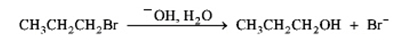
or
b.

Consider the reactions above.
a) Which reaction would be predicted to be faster?
b) Classify the reactions as SN1 or SN2?
c) Explain your answers to the questions above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
16
Draw the structure of the product of the following reaction.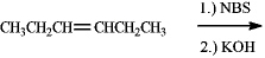
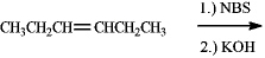

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
17
Instructions: Consider the pair of reactions below to answer the following question(s). 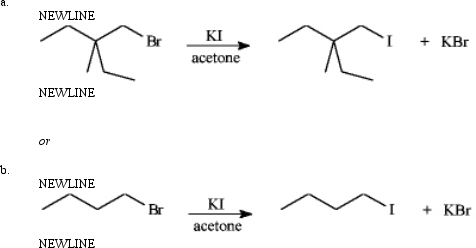
Refer to instructions.The nucleophile in these reactions is:
A)K+
B)alkyl group
C)Br-
D)I-

Refer to instructions.The nucleophile in these reactions is:
A)K+
B)alkyl group
C)Br-
D)I-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
18
Instructions: Circle the correct response in each set below.
Refer to instructions.The best nucleophile in a substitution reaction at a primary carbon.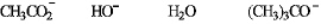
Refer to instructions.The best nucleophile in a substitution reaction at a primary carbon.
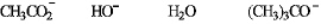

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
19
Instructions: Consider the pair of reactions below to answer the following question(s). 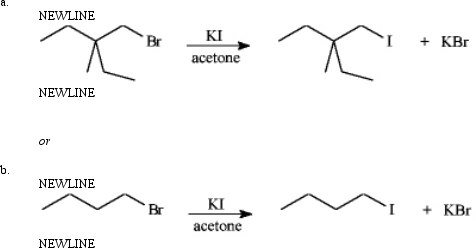
Refer to instructions.The solvent in these reactions is:
A)nonpolar aprotic
B)polar aprotic
C)polar protic
D)nonpolar protic

Refer to instructions.The solvent in these reactions is:
A)nonpolar aprotic
B)polar aprotic
C)polar protic
D)nonpolar protic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following would produce a mixture of products when treated under appropriate conditions with N-bromosuccinimide?
A)oct-4-ene
B)hept-1-ene
C)4,4-dimethylcyclopentene
D)4,5-dimethylcyclohexene
E)all of these produce a mixture of products
A)oct-4-ene
B)hept-1-ene
C)4,4-dimethylcyclopentene
D)4,5-dimethylcyclohexene
E)all of these produce a mixture of products

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the rate law for the E2 reaction of an alkyl halide (RX)with sodium ethoxide (NaOEt)in ethanol solvent (EtOH)?
A)rate = k[RX]
B)rate = k[RX]2
C)rate = k[RX][Na+]
D)rate = k[RX][OEt-]
E)rate = k[OEt-]
A)rate = k[RX]
B)rate = k[RX]2
C)rate = k[RX][Na+]
D)rate = k[RX][OEt-]
E)rate = k[OEt-]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the IUPAC name of the following compound? 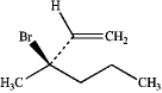
A)(R)-2-bromo-2-vinylpentane
B)(S)-2-bromo-2-vinylpentane
C)(S)-3-bromo-3-propylbut-1-ene
D)(R)-3-bromo-3-methylhex-1-ene

A)(R)-2-bromo-2-vinylpentane
B)(S)-2-bromo-2-vinylpentane
C)(S)-3-bromo-3-propylbut-1-ene
D)(R)-3-bromo-3-methylhex-1-ene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
23
The bromination of an alkene using N-bromosuccinimde involves
A)the formation of a free radical.
B)a chain reaction mechanism.
C)a resonance stabilized intermediate.
D)maintaining the double bond in the alkene.
E)all of these
A)the formation of a free radical.
B)a chain reaction mechanism.
C)a resonance stabilized intermediate.
D)maintaining the double bond in the alkene.
E)all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which mechanism is favored by the reaction of a secondary alkyl bromide with potassium t-butoxide?
A)SN1
B)SN2
C)E1
D)E1CB
E)E2
A)SN1
B)SN2
C)E1
D)E1CB
E)E2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following statements about an SN2 reaction is true?
A)There are two transition states.
B)There is one energy maximum.
C)The transition state can be isolated and studied.
D)For a carbon electrophile in the transition state,the hybridization of an sp3 carbon remains unchanged.
E)The reaction rate does not depend on the concentration of the electrophile.
A)There are two transition states.
B)There is one energy maximum.
C)The transition state can be isolated and studied.
D)For a carbon electrophile in the transition state,the hybridization of an sp3 carbon remains unchanged.
E)The reaction rate does not depend on the concentration of the electrophile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
26
Consider the following compound: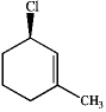
a)What is the IUPAC name of the compound?
a. (R)-1-chloro-3-methyl-2-cyclohexene
b. (S)-1-chloro-3-methyl-2-cyclohexene
c. (R)-3-chloro-1-methylcyclohexene
d. (S)-3-chloro-1-methylcyclohexene
b)How could this compound be used to produce a conjugated diene?
a. substitution
b. elimination
c. allylic free radical formation
d. either b or c

a)What is the IUPAC name of the compound?
a. (R)-1-chloro-3-methyl-2-cyclohexene
b. (S)-1-chloro-3-methyl-2-cyclohexene
c. (R)-3-chloro-1-methylcyclohexene
d. (S)-3-chloro-1-methylcyclohexene
b)How could this compound be used to produce a conjugated diene?
a. substitution
b. elimination
c. allylic free radical formation
d. either b or c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which is most reactive in an SN1 reaction? Explain your choice.
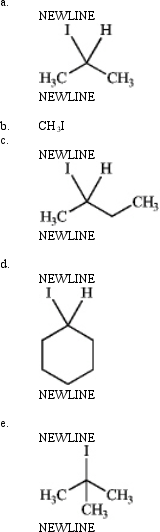


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements about an SN2 reaction is true?
A)the reaction occurs in two steps
B)rate = k[RX]
C)stabilization of R+ is important
D)the reaction causes racemization
E)the reaction is favored by aprotic solvents
A)the reaction occurs in two steps
B)rate = k[RX]
C)stabilization of R+ is important
D)the reaction causes racemization
E)the reaction is favored by aprotic solvents

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
29
Upon treatment with base,the following isotopically labeled compound gives rise to a major and a minor elimination product.Draw their structures and label each as major or minor.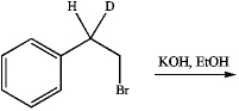


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
30
Instructions: Consider the reaction below to answer the following question(s). 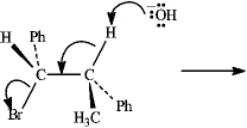
Refer to instructions.The mechanism of this reaction is:
A)SN1
B)SN2
C)E1
D)E2

Refer to instructions.The mechanism of this reaction is:
A)SN1
B)SN2
C)E1
D)E2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
31
Consider the following reaction: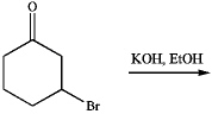
a)Draw the product of the reaction. How would you categorize this process mechanistically?
b)Which of the following spectral methods could be used to follow the progress of the reaction?
a. NMR spectroscopy
b. Mass spectrometry
c. Infrared spectroscopy
d. UV spectroscopy
e. all of these
f. a, b, and c only

a)Draw the product of the reaction. How would you categorize this process mechanistically?
b)Which of the following spectral methods could be used to follow the progress of the reaction?
a. NMR spectroscopy
b. Mass spectrometry
c. Infrared spectroscopy
d. UV spectroscopy
e. all of these
f. a, b, and c only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the preferred stereochemistry of the E2 elimination?
A)inversion
B)retention
C)antiperiplanar
D)synperiplanar
E)gauche
A)inversion
B)retention
C)antiperiplanar
D)synperiplanar
E)gauche

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
33
Predict the product of the following reaction: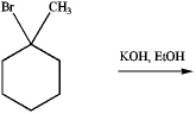


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following statements about an SN1 reaction is true?
A)the reaction occurs in one-step
B)there is no effect on reaction rate by nucleophile
C)primary alkyl halides react faster than secondary alkyl halides
D)the reaction proceeds with inversion of stereochemistry
E)the reaction is favored by aprotic solvents
A)the reaction occurs in one-step
B)there is no effect on reaction rate by nucleophile
C)primary alkyl halides react faster than secondary alkyl halides
D)the reaction proceeds with inversion of stereochemistry
E)the reaction is favored by aprotic solvents

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following represents the transition state of the SN2 reaction between methyl iodide and ammonia?
A)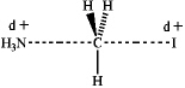
B)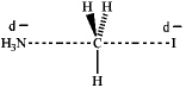
C)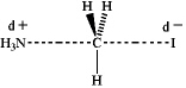
D)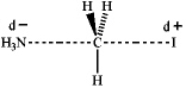
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which mechanism is favored by the reaction of a tertiary alkyl chloride with ethanol?
A)SN1
B)SN2
C)E1
D)E1CB
E)E2
A)SN1
B)SN2
C)E1
D)E1CB
E)E2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which conditions favor an efficient (fast,high yield)SN2 reaction between an appropriate alkyl halide and a nucleophile with a charge?
A)high concentration of a strong nucleophile,polar protic solvent
B)high concentration of a weak nucleophile,nonpolar solvent
C)low concentration of a strong nucleophile,polar aprotic solvent
D)low concentration of a weak nucleophile,nonpolar solvent
E)high concentration of a strong nucleophile,polar aprotic solvent
A)high concentration of a strong nucleophile,polar protic solvent
B)high concentration of a weak nucleophile,nonpolar solvent
C)low concentration of a strong nucleophile,polar aprotic solvent
D)low concentration of a weak nucleophile,nonpolar solvent
E)high concentration of a strong nucleophile,polar aprotic solvent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 37 في هذه المجموعة.
فتح الحزمة
k this deck








