Deck 3: Mass Relationships in Chemical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/194
العب
ملء الشاشة (f)
Deck 3: Mass Relationships in Chemical Reactions
1
If 0.274 moles of a substance weighs 62.5 g, what is the molar mass of the substance, in units of g/mol?
A)2.28 × 102 g/mol
B)1.71 × 101 g/mol
C)4.38 × 10-3 g/mol
D)2.17 × 102 g/mol
E)6.02 x 1023 g/mol
A)2.28 × 102 g/mol
B)1.71 × 101 g/mol
C)4.38 × 10-3 g/mol
D)2.17 × 102 g/mol
E)6.02 x 1023 g/mol
2.28 × 102 g/mol
2
An average atom of uranium (U)is approximately how many times heavier than an atom of potassium?
A)6.1 times
B)4.8 times
C)2.4 times
D)12.5 times
E)7.7 times
A)6.1 times
B)4.8 times
C)2.4 times
D)12.5 times
E)7.7 times
6.1 times
3
Boron obtained from borax deposits in Death Valley consists of two isotopes.They are boron-10 and boron-11 with atomic masses of 10.013 amu and 11.009 amu, respectively.The atomic mass of boron is 10.81 amu (see periodic table).Which isotope of boron is more abundant, boron-10 or boron-11?
A)Cannot be determined from data given
B)Neither, their abundances are the same.
C)Boron-10
D)Boron-11
A)Cannot be determined from data given
B)Neither, their abundances are the same.
C)Boron-10
D)Boron-11
Boron-11
4
Calculate the average atomic mass of silver using the following data: 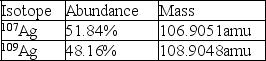
A)106.91 amu
B)108.00 amu
C)107.90 amu
D)107.87 amu
E)108.90 amu

A)106.91 amu
B)108.00 amu
C)107.90 amu
D)107.87 amu
E)108.90 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
5
Calculate the average atomic mass of lithium using the following data: 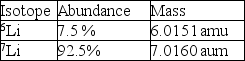
A)6.51 amu
B)6.02 amu
C)6.94 amu
D)7.02 amu
E)6.50 amu

A)6.51 amu
B)6.02 amu
C)6.94 amu
D)7.02 amu
E)6.50 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the average mass, in grams, of one Zn atom?
A)65.39 amu
B)65.39 g
C)3.94 x 1025g
D)1.09 x 10-22 g
E)1.661 x 10-24g
A)65.39 amu
B)65.39 g
C)3.94 x 1025g
D)1.09 x 10-22 g
E)1.661 x 10-24g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the average mass of one S atom?
A)32.07 g
B)32.07 amu
C)32.07 g/mol
D)5.32 x 10-23 amu
E)1.93 x 1025 g
A)32.07 g
B)32.07 amu
C)32.07 g/mol
D)5.32 x 10-23 amu
E)1.93 x 1025 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the mass of 3.50 x 1024 Ti atoms?
A)47.9 amu
B)47.9 g
C)5.81 g
D)278 g
E)5.81 amu
A)47.9 amu
B)47.9 g
C)5.81 g
D)278 g
E)5.81 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
9
The element oxygen consists of three naturally occurring isotopes: 16O, 17O, and 18O.The atomic mass of oxygen is 16.0 amu.What can be implied about the relative abundances of these isotopes?
A)More than 50% of all O atoms are 17O.
B)Almost all O atoms are 18O.
C)Almost all O atoms are 17O.
D)The isotopes all have the same abundance, i.e.33.3%.
E)The abundances of 17O and 18O are very small.
A)More than 50% of all O atoms are 17O.
B)Almost all O atoms are 18O.
C)Almost all O atoms are 17O.
D)The isotopes all have the same abundance, i.e.33.3%.
E)The abundances of 17O and 18O are very small.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the molecular mass of Br2 ?
A)79.90 amu
B)79.90 g
C)159.8 amu
D)159.8 g
E)2.65 x 10-22 amu
A)79.90 amu
B)79.90 g
C)159.8 amu
D)159.8 g
E)2.65 x 10-22 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
11
An atom of helium has a mass about four times greater than that of an atom of hydrogen.Which choice makes the correct comparison of the relative numbers of helium and hydrogen atoms in equal masses of the two elements?
A)There are about four times as many helium atoms as hydrogen atoms.
B)There are about two times as many helium atoms as hydrogen atoms.
C)The number of helium and hydrogen atoms is the same.
D)There are about half as many helium atoms as hydrogen atoms.
E)There are about one-fourth as many helium atoms as hydrogen atoms.
A)There are about four times as many helium atoms as hydrogen atoms.
B)There are about two times as many helium atoms as hydrogen atoms.
C)The number of helium and hydrogen atoms is the same.
D)There are about half as many helium atoms as hydrogen atoms.
E)There are about one-fourth as many helium atoms as hydrogen atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which one of the following does not represent 1.000 mol of the indicated substance?
A)6.022 × 1023 C atoms
B)26.00 g Fe
C)12.01 g C
D)65.39 g Zn
E)6.022 × 1023 Fe atoms
A)6.022 × 1023 C atoms
B)26.00 g Fe
C)12.01 g C
D)65.39 g Zn
E)6.022 × 1023 Fe atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many Br atoms are in 2.50 g of Br?
A)1.88x1022 Br atoms
B)1.51 x 1024 Br atoms
C)7.54 x 1021 Br atoms
D)6.02 x 1023 Br atoms
E)9.42 x 1021 Br atoms
A)1.88x1022 Br atoms
B)1.51 x 1024 Br atoms
C)7.54 x 1021 Br atoms
D)6.02 x 1023 Br atoms
E)9.42 x 1021 Br atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the mass of 4.50 x 1022 Cu atoms?
A)7.47 x 10-2 g
B)7.47 x 10-2 amu
C)4.75 g
D)63.55 amu
E)63.55 g
A)7.47 x 10-2 g
B)7.47 x 10-2 amu
C)4.75 g
D)63.55 amu
E)63.55 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the average mass of one Ca atom?
A)40.08 amu
B)40.08 g
C)40.08 g/mol
D)2.41 x 10-21 amu
E)2.41x1025 g
A)40.08 amu
B)40.08 g
C)40.08 g/mol
D)2.41 x 10-21 amu
E)2.41x1025 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the average mass, in grams, of one Rb atom?
A)6.02 x 1023 g
B)1.42 x 10-22g
C)5.15 x 1025 g
D)85.47 g
E)85.47 amu
A)6.02 x 1023 g
B)1.42 x 10-22g
C)5.15 x 1025 g
D)85.47 g
E)85.47 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
17
An atom of bromine has a mass about four times greater than that of an atom of neon.How many grams of neon will contain the same number of atoms as 1,000 g of bromine?
A)4 g Ne
B)250 g Ne
C)400 g Ne
D)1,000 g Ne
E)4,000 g Ne
A)4 g Ne
B)250 g Ne
C)400 g Ne
D)1,000 g Ne
E)4,000 g Ne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
18
One mole of iron
A)is heavier than one mole of lead (Pb).
B)is 77.0 g of iron.
C)is 26.0 g of iron.
D)weighs the same as one mole of lead.
E)None of the above.
A)is heavier than one mole of lead (Pb).
B)is 77.0 g of iron.
C)is 26.0 g of iron.
D)weighs the same as one mole of lead.
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
19
There are two stable isotopes of chlorine: chlorine-35, with a mass of 34.968853 amu; and chlorine-37, with a mass of 36.965903.Given that the average atomic mass of a chlorine atom is 35.45 amu, which of the following statements is true?
A)Chlorine contains almost exclusively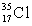 with very little
with very little 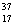 Cl.
Cl.
B)Chlorine contains more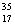 Cl than
Cl than 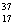 Cl.
Cl.
C)Chlorine contains roughly equal amounts of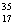 Cl and
Cl and 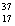 Cl.
Cl.
D)Chlorine contains more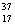 Cl than
Cl than 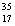 Cl.
Cl.
E)Chlorine contains almost exclusively of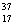 Cl, with very little
Cl, with very little 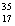 Cl.
Cl.
A)Chlorine contains almost exclusively
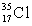 with very little
with very little 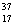 Cl.
Cl.B)Chlorine contains more
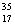 Cl than
Cl than 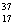 Cl.
Cl.C)Chlorine contains roughly equal amounts of
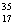 Cl and
Cl and 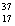 Cl.
Cl.D)Chlorine contains more
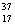 Cl than
Cl than 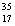 Cl.
Cl.E)Chlorine contains almost exclusively of
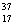 Cl, with very little
Cl, with very little 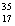 Cl.
Cl.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
20
An atom of bromine has a mass about four times greater than that of an atom of neon.Which choice makes the correct comparison of the relative numbers of bromine and neon atoms in 1,000 g of each element?
A)The number of bromine and neon atoms is the same.
B)There are one thousand times as many bromine atoms as neon atoms.
C)There are one thousand times as many neon atoms as bromine atoms.
D)There are four times as many neon atoms as bromine atoms.
E)There are four times as many bromine atoms as neon atoms.
A)The number of bromine and neon atoms is the same.
B)There are one thousand times as many bromine atoms as neon atoms.
C)There are one thousand times as many neon atoms as bromine atoms.
D)There are four times as many neon atoms as bromine atoms.
E)There are four times as many bromine atoms as neon atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
21
Calculate the number of moles of xenon in 12.0 g of xenon.
A)1.00 mol
B)0.0457 mol
C)0.183 mol
D)7.62 × 10-3 mol
E)0.0914 mol
A)1.00 mol
B)0.0457 mol
C)0.183 mol
D)7.62 × 10-3 mol
E)0.0914 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the molecular mass of nicotine, C10H14N2?
A)27.03 amu
B)148.22 amu
C)149.13 amu
D)81.12 amu
E)162.23 amu
A)27.03 amu
B)148.22 amu
C)149.13 amu
D)81.12 amu
E)162.23 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
23
Determine the number of moles of aluminum in 96.7 g of Al.
A)0.279 mol
B)3.58 mol
C)7.43 mol
D)4.21 mol
E)6.02 × 1023 mol
A)0.279 mol
B)3.58 mol
C)7.43 mol
D)4.21 mol
E)6.02 × 1023 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many moles of CF4 are there in 171 g of CF4?
A)0.51 mol
B)1.94 mol
C)4.07 mol
D)88.0 mol
E)171 mol
A)0.51 mol
B)1.94 mol
C)4.07 mol
D)88.0 mol
E)171 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following NH3 samples contains the greatest number of moles of NH3?
A)0.356 moles NH3
B)4.65 x 1023 NH3 molecules
C)6.78 x 1022 NH3 molecules
D)8.90g NH3
E)6.78 x 101g NH3
A)0.356 moles NH3
B)4.65 x 1023 NH3 molecules
C)6.78 x 1022 NH3 molecules
D)8.90g NH3
E)6.78 x 101g NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
26
How many F atoms are in 5.54 g of F2?
A)6.02 × 1023 atoms
B)0.146 atoms
C)0.292 atoms
D)8.78 × 1022 atoms
E)1.76 × 1023 atoms
A)6.02 × 1023 atoms
B)0.146 atoms
C)0.292 atoms
D)8.78 × 1022 atoms
E)1.76 × 1023 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
27
How many moles of NH3 are there in 77.5 g of NH3?
A)0.220 mol
B)4.55 mol
C)14.0 mol
D)1.31 × 103 mol
E)None of the above.
A)0.220 mol
B)4.55 mol
C)14.0 mol
D)1.31 × 103 mol
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
28
How many C atoms are in 5.50 g of C?
A)5.01 x 1022 C atoms
B)3.31 x 1024 C atoms
C)6.02 x 1023 C atoms
D)2.76 x 1023 C atoms
E)5.50 x 1023 C atoms
A)5.01 x 1022 C atoms
B)3.31 x 1024 C atoms
C)6.02 x 1023 C atoms
D)2.76 x 1023 C atoms
E)5.50 x 1023 C atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
29
Calculate the molecular mass of menthol, C10H20O.
A)156.26 amu
B)140.26 amu
C)29.02 amu
D)48.17 amu
E)137.11 amu
A)156.26 amu
B)140.26 amu
C)29.02 amu
D)48.17 amu
E)137.11 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
30
Calculate the molar mass of Ba(NO3)2.
A)199.3 g/mol
B)323.3 g/mol
C)247.3 g/mol
D)261.3 g/mol
E)398.6 g/mol
A)199.3 g/mol
B)323.3 g/mol
C)247.3 g/mol
D)261.3 g/mol
E)398.6 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
31
A silver wire has a diameter of 0.500 mm.What length of this wire contains exactly 1.00 mol of silver? (density of Ag = 10.5 g/cm3)
A)52.3 m
B)222 m
C)13.1 m
D)2.01 m
E)890 m
A)52.3 m
B)222 m
C)13.1 m
D)2.01 m
E)890 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
32
How many Cl atoms are in 0.0728 g of PCl3?
A)4.38 x 1022 Cl atoms
B)1.32 x 1023 Cl atoms
C)3.19 x 1020 Cl atoms
D)9.58 x 1020 Cl atoms
E)1.81 x 1024 Cl atoms
A)4.38 x 1022 Cl atoms
B)1.32 x 1023 Cl atoms
C)3.19 x 1020 Cl atoms
D)9.58 x 1020 Cl atoms
E)1.81 x 1024 Cl atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following CO2 samples contains the greatest number of moles of CO2?
A)3.5 moles CO2
B)3.21 x 1023 CO2 molecules
C)4.50 x 1022 CO2 molecules
D)5.60 g CO2
E)3.19 x 101 g CO2
A)3.5 moles CO2
B)3.21 x 1023 CO2 molecules
C)4.50 x 1022 CO2 molecules
D)5.60 g CO2
E)3.19 x 101 g CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the molar mass of acetaminophen, C8H9NO2?
A)151.16 g/mol
B)43.03 g/mol
C)67.09 g/mol
D)143.10 g/mol
E)135.16 g/mol
A)151.16 g/mol
B)43.03 g/mol
C)67.09 g/mol
D)143.10 g/mol
E)135.16 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
35
A copper wire has a diameter of 2.00 mm.What length of this wire contains exactly 1.00 mol of copper? (density of Cu = 8.92 g/cm3)
A)0.178 m
B)0.567 m
C)180 m
D)45.1 m
E)2.27 m
A)0.178 m
B)0.567 m
C)180 m
D)45.1 m
E)2.27 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
36
Calculate the molar mass of (NH4)2SO4
A)114.11 g/mol
B)228.22 g/mol
C)118.14 g/mol
D)63.09 g/mol
E)132.15 g/mol
A)114.11 g/mol
B)228.22 g/mol
C)118.14 g/mol
D)63.09 g/mol
E)132.15 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following samples contains the greatest number of atoms?
A)100 g of Pb
B)2.0 mole of Ar
C)0.1 mole of Fe
D)5 g of He
E)20 million O2 molecules
A)100 g of Pb
B)2.0 mole of Ar
C)0.1 mole of Fe
D)5 g of He
E)20 million O2 molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
38
How many O atoms are in 4.39 g of CO2?
A)5.29 x 1024 O atoms
B)1.03 x 1022 O atoms
C)1.65 x 1023 O atoms
D)6.01 x 1022 O atoms
E)1.20 x 1023 O atoms
A)5.29 x 1024 O atoms
B)1.03 x 1022 O atoms
C)1.65 x 1023 O atoms
D)6.01 x 1022 O atoms
E)1.20 x 1023 O atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
39
A gold wire has a diameter of 1.00 mm.What length of this wire contains exactly 1.00 mol of gold? (density of Au = 17.0 g/cm3)
A)2630 m
B)3.69 m
C)251 m
D)14.8 m
E)62.7 m
A)2630 m
B)3.69 m
C)251 m
D)14.8 m
E)62.7 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
40
Calculate the number of moles of cesium in 50.0 g of cesium.
A)0.376 mol
B)0.357 mol
C)2.66 mol
D)2.80 mol
E)0.0200 mol
A)0.376 mol
B)0.357 mol
C)2.66 mol
D)2.80 mol
E)0.0200 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
41
How many moles of C are in 1.22 moles of C6H12O6?
A)7.32 moles C
B)87.9 moles C
C)1.22 moles C
D)14.7 moles C
E)2.44 moles C
A)7.32 moles C
B)87.9 moles C
C)1.22 moles C
D)14.7 moles C
E)2.44 moles C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
42
Calculate the mass of 4.50 moles of Ca3PO4
A)215 g
B)968 g
C)0.0209 g
D)87.1 g
E)392 g
A)215 g
B)968 g
C)0.0209 g
D)87.1 g
E)392 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
43
Calculate the mass of 3.00 moles of CF2Cl2.
A)3.00 g
B)174 g
C)363 g
D)1.81 × 1024 g
E)40.3 g
A)3.00 g
B)174 g
C)363 g
D)1.81 × 1024 g
E)40.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
44
How many moles of H are in 4.56 moles of NH2NH2?
A)4.52 moles H
B)4.56 moles H
C)9.12 moles H
D)18.39 moles H
E)18.24 moles H
A)4.52 moles H
B)4.56 moles H
C)9.12 moles H
D)18.39 moles H
E)18.24 moles H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
45
How many sulfur atoms are there in 21.0 g of Al2S3?
A)8.42 × 1022 atoms
B)2.53 × 1023 atoms
C)2.14 × 1023 atoms
D)6.02 × 1023 atoms
E)6.30 × 1026 atoms
A)8.42 × 1022 atoms
B)2.53 × 1023 atoms
C)2.14 × 1023 atoms
D)6.02 × 1023 atoms
E)6.30 × 1026 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many molecules are there in 8.0 g of ozone, O3?
A)3.0 molecules
B)3.6 × 1024 molecules
C)1.0 × 1023 molecules
D)3.0 × 1023 molecules
E)6.0 × 1023 molecules
A)3.0 molecules
B)3.6 × 1024 molecules
C)1.0 × 1023 molecules
D)3.0 × 1023 molecules
E)6.0 × 1023 molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
47
How many moles of HCl are represented by 1.0 × 1019 HCl molecules?
A)1.7 × 10-5 mol
B)1.5 × 10-3 mol
C)1.0 × 1019 mol
D)37 mol
E)6.0 × 104 mol
A)1.7 × 10-5 mol
B)1.5 × 10-3 mol
C)1.0 × 1019 mol
D)37 mol
E)6.0 × 104 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
48
How many moles of Cl atoms are there in 65.2 g CHCl3?
A)0.548 mol
B)1.09 mol
C)3.30 × 1023 mol
D)1.64 mol
E)3.00 mol
A)0.548 mol
B)1.09 mol
C)3.30 × 1023 mol
D)1.64 mol
E)3.00 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
49
Calculate the mass of 0.00456 moles of (NH4)2SO4
A)132 g
B)3.45 x 10-5 g
C)114 g
D)0.603 g
E)0.520 g
A)132 g
B)3.45 x 10-5 g
C)114 g
D)0.603 g
E)0.520 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the mass of 0.0250 mol of P2O5?
A)35.5 g
B)5676 g
C)0.0250 g
D)1.51 × 1022 g
E)3.55 g
A)35.5 g
B)5676 g
C)0.0250 g
D)1.51 × 1022 g
E)3.55 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
51
Calculate the mass of O in 4.36 g of Cl2O7?
A)30.5 g O
B)48.8 g O
C)11.2 g O
D)69.8 g O
E)2.67 g O
A)30.5 g O
B)48.8 g O
C)11.2 g O
D)69.8 g O
E)2.67 g O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
52
How many O atoms are there in 51.4 g CaSO4?
A)4.00
B)2.40 × 1024
C)1.13
D)9.09 × 1023
E)2.28 × 1023
A)4.00
B)2.40 × 1024
C)1.13
D)9.09 × 1023
E)2.28 × 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
53
How many moles of O are in 2.45 moles of H2CO3?
A)2.45 moles O
B)39.2 moles O
C)118 moles O
D)7.35 moles O
E)0.459 moles O
A)2.45 moles O
B)39.2 moles O
C)118 moles O
D)7.35 moles O
E)0.459 moles O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
54
Formaldehyde has the formula CH2O.How many molecules are there in 0.11 g of formaldehyde?
A)6.1 × 10-27
B)3.7 × 10-3
C)4.0
D)2.2 × 1021
E)6.6 × 1022
A)6.1 × 10-27
B)3.7 × 10-3
C)4.0
D)2.2 × 1021
E)6.6 × 1022

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
55
How many sulfur atoms are present in 25.6 g of Al2(S2O3)3?
A)0.393
B)6.00
C)3.95 × 1022
D)7.90 × 1022
E)2.37 × 1023
A)0.393
B)6.00
C)3.95 × 1022
D)7.90 × 1022
E)2.37 × 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
56
How many moles of O atoms are in 25.7 g of CaSO4?
A)0.189 mol
B)0.755 mol
C)4.00 mol
D)1.14 × 1023 mol
E)4.55 × 1023 mol
A)0.189 mol
B)0.755 mol
C)4.00 mol
D)1.14 × 1023 mol
E)4.55 × 1023 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
57
The molecular formula of aspirin is C9H8O4.How many aspirin molecules are present in one 500-milligram tablet?
A)2.77 molecules
B)2.77 × 10-3 molecules
C)1.67 × 1024 molecules
D)1.67 × 1021 molecules
E)None of these is correct.
A)2.77 molecules
B)2.77 × 10-3 molecules
C)1.67 × 1024 molecules
D)1.67 × 1021 molecules
E)None of these is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
58
How many fluorine atoms are there in 65 g of CF4?
A)0.74 atoms
B)3.0 atoms
C)4.5 × 1023 atoms
D)1.8 × 1024 atoms
E)2.4 × 1023 atoms
A)0.74 atoms
B)3.0 atoms
C)4.5 × 1023 atoms
D)1.8 × 1024 atoms
E)2.4 × 1023 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
59
How many carbon atoms are there in 15 lbs of sugar, C12H22O11?
A)4.1 x 1028 C atoms
B)1.2 x 1026 C atoms
C)1.4 x 1026 C atoms
D)2.6 x 1022 C atoms
E)3.2 x 1023 C atoms
A)4.1 x 1028 C atoms
B)1.2 x 1026 C atoms
C)1.4 x 1026 C atoms
D)2.6 x 1022 C atoms
E)3.2 x 1023 C atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
60
How many sodium atoms are there in 6.0 g of Na3N?
A)3.6 × 1024 atoms
B)4.6 × 1022 atoms
C)1.3 × 1023 atoms
D)0.22 atoms
E)0.072 atoms
A)3.6 × 1024 atoms
B)4.6 × 1022 atoms
C)1.3 × 1023 atoms
D)0.22 atoms
E)0.072 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
61
A compound with an empirical formula of C2H2Br3 has a molar mass of 531.47 g/mol.What is the molecular formula?
A)C2H2Br3
B)C4H4Br6
C)CHBr
D)C4H4Br3
E)C6H6Br9
A)C2H2Br3
B)C4H4Br6
C)CHBr
D)C4H4Br3
E)C6H6Br9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
62
The empirical formula of a compound of uranium and fluorine that is composed of 67.6% uranium and 32.4% fluorine is
A)U2F
B)U3F4
C)UF4
D)UF6
E)UF8
A)U2F
B)U3F4
C)UF4
D)UF6
E)UF8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
63
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% C, 7.80% H, and 45.72% Cl.Find the molecular formula for the compound.
A)CHCl
B)C9H18Cl3
C)C6H12Cl
D)C6H12Cl2
E)C3H6Cl
A)CHCl
B)C9H18Cl3
C)C6H12Cl
D)C6H12Cl2
E)C3H6Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
64
The percent composition by mass of a compound is 76.0% C, 12.8% H, and 11.2% O.The molar mass of this compound is 284.5 g/mol.What is the molecular formula of the compound?
A)C10H6O
B)C9H18O
C)C16H28O4
D)C20H12O2
E)C18H36O2
A)C10H6O
B)C9H18O
C)C16H28O4
D)C20H12O2
E)C18H36O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
65
The mineral orpiment, having the empirical formula As2S3, was used in ancient times as a cosmetic.What mass of arsenic is present in 5.0 g of orpiment? [Given: naturally occurring arsenic is all arsenic-75; assume that all naturally occurring sulfur is sulfur-32 (only approximately true)]
A)0.61 g
B)3.0 g
C)1.5 g
D)2.0 g
E)3.5 g
A)0.61 g
B)3.0 g
C)1.5 g
D)2.0 g
E)3.5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
66
An unknown compound with a molar mass of 223.94 g/mol consists of 32.18% C, 4.50% H, and 63.32% Cl.Find the molecular formula for the compound.
A)CHCl
B)C6H10Cl4
C)C3H5Cl2
D)C9H15Cl6
E)C6H10Cl2
A)CHCl
B)C6H10Cl4
C)C3H5Cl2
D)C9H15Cl6
E)C6H10Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the mass of 8.25 × 1019 UF6 molecules?
A)352 g
B)0.0482 g
C)1.37 × 10-4 g
D)2.90 × 1022 g
E)8.25 × 1019 g
A)352 g
B)0.0482 g
C)1.37 × 10-4 g
D)2.90 × 1022 g
E)8.25 × 1019 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
68
Zircon is a mineral with the empirical formula ZrSiO4.If all the zirconium is 90Zr, all the silicon is 28Si, and all the oxygen is 16O, what mass of oxygen is present in 10.g of zircon?
A)0.88 g
B)1.2 g
C)1.8 g
D)3.5 g
E)5.4 g
A)0.88 g
B)1.2 g
C)1.8 g
D)3.5 g
E)5.4 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
69
Calculate the mass of C in 5.46 g of C2H4?
A)0.455 g C
B)4.68 g C
C)65.6 g C
D)13.1 g C
E)24.0 g C
A)0.455 g C
B)4.68 g C
C)65.6 g C
D)13.1 g C
E)24.0 g C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
70
Calculate the mass of N in 2.34 g of N2H4?
A)4.68 g N
B)65.6 g N
C)28.02 g N
D)2.05 g N
E)2.34 g N
A)4.68 g N
B)65.6 g N
C)28.02 g N
D)2.05 g N
E)2.34 g N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
71
The mineral pyrolusite is a compound of 55Mn and 16O.If 63% of the mass of pyrolusite is due to manganese, what is the empirical formula of pyrolusite?
A)MnO
B)Mn2O
C)Mn2O2
D)MnO2
E)none of these
A)MnO
B)Mn2O
C)Mn2O2
D)MnO2
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the mass of 0.55 mole of C6H6?
A)78.11 g
B)78.11 amu
C)42.96 g
D)42.96 amu
E)7.04 x 10-3 g
A)78.11 g
B)78.11 amu
C)42.96 g
D)42.96 amu
E)7.04 x 10-3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
73
The mineral hausmannite is a compound of 55Mn and 16O.If 72% of the mass of hausmannite is due to manganese, what is the empirical formula of hausmannite?
A)MnO
B)Mn3O
C)Mn3O4
D)Mn4O3
E)MnO3
A)MnO
B)Mn3O
C)Mn3O4
D)Mn4O3
E)MnO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
74
A compound with an empirical formula of C2H3Br2 has a molar mass of 373.69 g/mol.What is the molecular formula?
A)C2H3Br2
B)CHBr
C)C6H9Br6
D)C4H6Br2
E)C4H6Br4
A)C2H3Br2
B)CHBr
C)C6H9Br6
D)C4H6Br2
E)C4H6Br4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
75
A mass spectrometer works by ionizing atoms or molecules, and then accelerating them through oppositely charged plates.The mass is obtained by
A)measuring the force of impact on a detecting screen, and then calculating the mass using force = mass × acceleration.
B)suspending the ions in an applied electric field, and then calculating mass by the setting the downward gravitational force equal to the upward electrostatic force.
C)measuring the magnitude of deflection as the ions pass through a magnetic field to obtain the charge-to-mass ratio, and then calculating the mass from that ratio.
D)measuring the time it takes for the ions to hit the detector at a known distance to calculate the acceleration, and then calculating mass from force = mass × acceleration.
A)measuring the force of impact on a detecting screen, and then calculating the mass using force = mass × acceleration.
B)suspending the ions in an applied electric field, and then calculating mass by the setting the downward gravitational force equal to the upward electrostatic force.
C)measuring the magnitude of deflection as the ions pass through a magnetic field to obtain the charge-to-mass ratio, and then calculating the mass from that ratio.
D)measuring the time it takes for the ions to hit the detector at a known distance to calculate the acceleration, and then calculating mass from force = mass × acceleration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
76
Calculate the mass of 4.50 moles of chlorine gas, Cl2.
A)6.34 × 10-2 g
B)4.50 g
C)15.7 g
D)160.g
E)319 g
A)6.34 × 10-2 g
B)4.50 g
C)15.7 g
D)160.g
E)319 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
77
How many grams of nitrogen are there in 7.5 g of Ca(NO3)2?
A)0.64 g
B)1.3 g
C)0.15 g
D)1.2 g
E)2.3 g
A)0.64 g
B)1.3 g
C)0.15 g
D)1.2 g
E)2.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
78
What is the mass of 3.00 moles of ethanol, C2H6O?
A)4.99 × 10-24 g
B)138 g
C)6.52 × 10-2 g
D)50.0 g
E)1.81 × 1024 g
A)4.99 × 10-24 g
B)138 g
C)6.52 × 10-2 g
D)50.0 g
E)1.81 × 1024 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
79
A compound with an empirical formula of C2H4Br has a molar mass of 215.90 g/mol.What is the molecular formula?
A)C4H8Br2
B)C2H4Br
C)CHBr
D)C6H12Br3
E)C4H8Br
A)C4H8Br2
B)C2H4Br
C)CHBr
D)C6H12Br3
E)C4H8Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
80
The mineral manganosite is a compound of 55Mn and 16O.If 77% of the mass of manganosite is due to manganese, what is the empirical formula of manganosite?
A)MnO
B)Mn2O
C)Mn2O2
D)MnO2
E)none of these
A)MnO
B)Mn2O
C)Mn2O2
D)MnO2
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck








