Deck 23: Nuclear Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/112
العب
ملء الشاشة (f)
Deck 23: Nuclear Chemistry
1
Alpha particles are identical to
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
helium nuclei.
2
What is the missing symbol in this plutonium fission reaction? 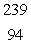 Pu +
Pu +  n
n  ______ +
______ + 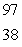 Sr + 3
Sr + 3 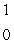 n
n
A)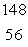 Ba
Ba
B)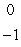
C)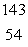 Xe
Xe
D)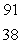 Sr
Sr
E)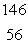 Ba
Ba
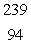 Pu +
Pu +  n
n 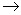 ______ +
______ + 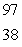 Sr + 3
Sr + 3 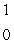 n
nA)
 Ba
BaB)
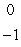
C)
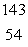 Xe
XeD)
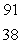 Sr
SrE)
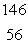 Ba
Ba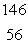 Ba
Ba 3
When atoms of beryllium-9 are bombarded with alpha particles, neutrons are produced.What new isotope is also formed? 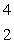 He +
He + 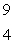 Be
Be 
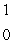 n + _____
n + _____
A) C
C
B)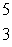 Li
Li
C)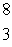 Li
Li
D)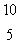 B
B
E)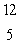 B
B
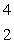 He +
He + 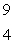 Be
Be 
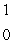 n + _____
n + _____A)
 C
CB)
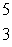 Li
LiC)
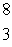 Li
LiD)
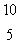 B
BE)
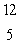 B
B C
C 4
Radium-226 decays by alpha emission. What is its decay product?
A)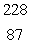 Fr
Fr
B)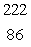 Rn
Rn
C)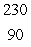 Th
Th
D)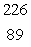 Ac
Ac
E)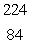 Po
Po
A)
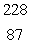 Fr
FrB)
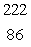 Rn
RnC)
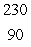 Th
ThD)
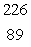 Ac
AcE)
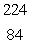 Po
Po
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
5
A radioisotope decays to give an alpha particle and Pb-208. What was the original element?
A)Se
B)Bi
C)Po
D)Hg
E)Rn
A)Se
B)Bi
C)Po
D)Hg
E)Rn

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
6
As a result of beta decay, the product nucleus is
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
7
The only stable isotope of aluminum is aluminum-27. What type of radioactive decay should be expected from 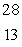 Al?
Al?
A)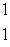 H
H
B)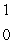 n
n
C)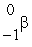
D)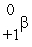
E)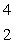 He
He
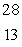 Al?
Al?A)
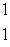 H
HB)
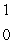 n
nC)
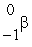
D)
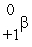
E)
 He
He
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
8
The only stable isotope of iodine is iodine-127. Predict the mode of decay of 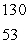 I.
I.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
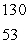 I.
I.A)alpha emission
B)beta emission
C)positron emission
D)electron capture

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
9
How many neutrons and protons (nucleons)does an atom with the symbol  have?
have?
A)33
B)16
C)49
D)None of the above.
 have?
have?A)33
B)16
C)49
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the nuclear binding energy per nucleon, in joules, for ![<strong>What is the nuclear binding energy per nucleon, in joules, for Mg (atomic mass 24.985839 amu). [Data: H (atomic mass)= 1.007825 amu; n (mass)= 1.008665 amu; 1 kg = 6.022 \times 10<sup>26</sup> amu; c = 3.00 \times 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 \times 10<sup>-11</sup> J/nucleon C)1.32 \times 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d0_e3fd_a2ab_951f73d4de35_TB3245_11.jpg) Mg (atomic mass 24.985839 amu). [Data:
Mg (atomic mass 24.985839 amu). [Data: ![<strong>What is the nuclear binding energy per nucleon, in joules, for Mg (atomic mass 24.985839 amu). [Data: H (atomic mass)= 1.007825 amu; n (mass)= 1.008665 amu; 1 kg = 6.022 \times 10<sup>26</sup> amu; c = 3.00 \times 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 \times 10<sup>-11</sup> J/nucleon C)1.32 \times 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_0b0e_a2ab_0b995ce574ff_TB3245_11.jpg) H (atomic mass)= 1.007825 amu;
H (atomic mass)= 1.007825 amu; ![<strong>What is the nuclear binding energy per nucleon, in joules, for Mg (atomic mass 24.985839 amu). [Data: H (atomic mass)= 1.007825 amu; n (mass)= 1.008665 amu; 1 kg = 6.022 \times 10<sup>26</sup> amu; c = 3.00 \times 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 \times 10<sup>-11</sup> J/nucleon C)1.32 \times 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_0b0f_a2ab_9bb5e486134d_TB3245_11.jpg) n (mass)= 1.008665 amu; 1 kg = 6.022 1026 amu; c = 3.00 108 m/s]
n (mass)= 1.008665 amu; 1 kg = 6.022 1026 amu; c = 3.00 108 m/s]
A)0.22076 J/nucleon
B)3.30 10-11 J/nucleon
C)1.32 10-12 J/nucleon
D)0.999 J/nucleon
E)None of the above.
![<strong>What is the nuclear binding energy per nucleon, in joules, for Mg (atomic mass 24.985839 amu). [Data: H (atomic mass)= 1.007825 amu; n (mass)= 1.008665 amu; 1 kg = 6.022 \times 10<sup>26</sup> amu; c = 3.00 \times 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 \times 10<sup>-11</sup> J/nucleon C)1.32 \times 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d0_e3fd_a2ab_951f73d4de35_TB3245_11.jpg) Mg (atomic mass 24.985839 amu). [Data:
Mg (atomic mass 24.985839 amu). [Data: ![<strong>What is the nuclear binding energy per nucleon, in joules, for Mg (atomic mass 24.985839 amu). [Data: H (atomic mass)= 1.007825 amu; n (mass)= 1.008665 amu; 1 kg = 6.022 \times 10<sup>26</sup> amu; c = 3.00 \times 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 \times 10<sup>-11</sup> J/nucleon C)1.32 \times 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_0b0e_a2ab_0b995ce574ff_TB3245_11.jpg) H (atomic mass)= 1.007825 amu;
H (atomic mass)= 1.007825 amu; ![<strong>What is the nuclear binding energy per nucleon, in joules, for Mg (atomic mass 24.985839 amu). [Data: H (atomic mass)= 1.007825 amu; n (mass)= 1.008665 amu; 1 kg = 6.022 \times 10<sup>26</sup> amu; c = 3.00 \times 10<sup>8</sup> m/s]</strong> A)0.22076 J/nucleon B)3.30 \times 10<sup>-11</sup> J/nucleon C)1.32 \times 10<sup>-12</sup> J/nucleon D)0.999 J/nucleon E)None of the above.](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_0b0f_a2ab_9bb5e486134d_TB3245_11.jpg) n (mass)= 1.008665 amu; 1 kg = 6.022 1026 amu; c = 3.00 108 m/s]
n (mass)= 1.008665 amu; 1 kg = 6.022 1026 amu; c = 3.00 108 m/s]A)0.22076 J/nucleon
B)3.30 10-11 J/nucleon
C)1.32 10-12 J/nucleon
D)0.999 J/nucleon
E)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
11
When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced. The particular isotope formed is 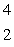 He +
He + 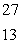 Al
Al 
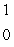 n + ____
n + ____
A)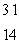 Si
Si
B)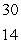 Si
Si
C)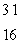 S
S
D)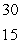 P
P
E)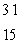 P
P
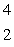 He +
He + 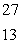 Al
Al 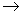
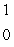 n + ____
n + ____A)
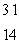 Si
SiB)
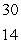 Si
SiC)
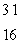 S
SD)
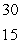 P
PE)
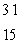 P
P
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
12
Predict the other product of the following nuclear transformation. 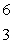 Li +
Li +  n
n  ____ +
____ + 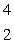 He
He
A)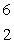 He
He
B)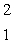 H
H
C)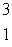 H
H
D)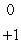
E)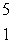 H
H
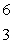 Li +
Li + 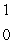 n
n  ____ +
____ + 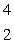 He
HeA)
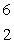 He
HeB)
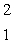 H
HC)
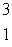 H
HD)
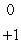
E)
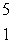 H
H
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
13
The isotope with the greatest nuclear binding energy per nucleon is
A)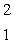 H
H
B)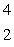 He
He
C)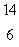 C
C
D)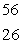 Fe
Fe
E)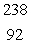 U
U
A)
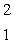 H
HB)
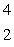 He
HeC)
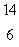 C
CD)
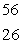 Fe
FeE)
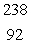 U
U
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
14
Find the nuclear binding enrgy of potassium-40 (atomic mass = 39.9632591 amu)in units of joules per nucleon.[Data: neutron mass = 1.674928 10-24 g; proton mass = 1.672623 10-24g; electron mass = 9.109387 10-28 g; NA = 6.0221367 1023 /mol; c = 2.99792458 108 m/s]
A)1.37 10-12 J/nucleon
B)5.48 10-11 J/nucleon
C)5.64 10-11 J/nucleon
D)1.41 10-12 J/nucleon
E)2.97 10-12 J/nucleon
A)1.37 10-12 J/nucleon
B)5.48 10-11 J/nucleon
C)5.64 10-11 J/nucleon
D)1.41 10-12 J/nucleon
E)2.97 10-12 J/nucleon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
15
Sulfur-35 decays by beta emission. The decay product is
A)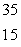 P
P
B) S
S
C)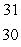 Si
Si
D)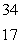 Cl
Cl
E) Cl
Cl
A)
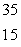 P
PB)
 S
SC)
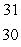 Si
SiD)
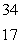 Cl
ClE)
 Cl
Cl
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
16
The energy equivalent of 1 amu is
A)5.0 10-19 J
B)5.4 1043 J
C)6.6 109 J
D)1.5 10-10 J
A)5.0 10-19 J
B)5.4 1043 J
C)6.6 109 J
D)1.5 10-10 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
17
A typical radius of an atomic nucleus is about
A)100 µm
B)5000 mm
C)100 nm
D)5 10-3 pm
E)500 pm
A)100 µm
B)5000 mm
C)100 nm
D)5 10-3 pm
E)500 pm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
18
In the uranium-238 decay series there are eight radioisotopes starting with 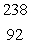 U that decay by alpha emission, and six radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
U that decay by alpha emission, and six radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
A)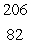 Pb
Pb
B)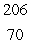 Yb
Yb
C)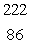 Rn
Rn
D)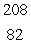 Pb
Pb
E)none of these
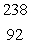 U that decay by alpha emission, and six radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
U that decay by alpha emission, and six radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product isA)
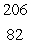 Pb
PbB)
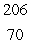 Yb
YbC)
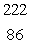 Rn
RnD)
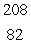 Pb
PbE)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
19
Beta particles are identical to
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
20
In the 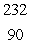 Th decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
Th decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
A)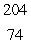 W
W
B)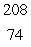 W
W
C)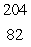 Pb
Pb
D)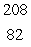 Pb
Pb
E)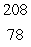 Pt
Pt
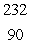 Th decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
Th decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product isA)
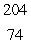 W
WB)
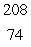 W
WC)
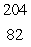 Pb
PbD)
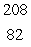 Pb
PbE)
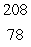 Pt
Pt
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
21
A rock contains 0.37 mg of Pb-206 and 0.95 mg of U-238. Approximately how many U-238 atoms were in the rock when it was formed billions of years ago? (The half life for 238U 206Pb is 4.5 109 yr.)
A)1.32 atoms
B)5.8 10-6 atoms
C)2.4 1018 atoms
D)3.5 1018 atoms
E)3.5 1021 atoms
A)1.32 atoms
B)5.8 10-6 atoms
C)2.4 1018 atoms
D)3.5 1018 atoms
E)3.5 1021 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
22
Determine how much energy is released when thorium-230 decays according to ![<strong>Determine how much energy is released when thorium-230 decays according to . [Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]</strong> A)3.98 \times 10<sup>9</sup> kJ/mol B)4.60 \times 10<sup>8</sup> kJ/mol C)7.20 \times 10<sup>11</sup> kJ/mol D)4.90 \times 10<sup>9</sup> kJ/mol E)7.15 \times 10<sup>11</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_8044_a2ab_f5e5d54af373_TB3245_11.jpg) . [Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]
. [Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]
A)3.98 109 kJ/mol
B)4.60 108 kJ/mol
C)7.20 1011 kJ/mol
D)4.90 109 kJ/mol
E)7.15 1011 kJ/mol
![<strong>Determine how much energy is released when thorium-230 decays according to . [Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]</strong> A)3.98 \times 10<sup>9</sup> kJ/mol B)4.60 \times 10<sup>8</sup> kJ/mol C)7.20 \times 10<sup>11</sup> kJ/mol D)4.90 \times 10<sup>9</sup> kJ/mol E)7.15 \times 10<sup>11</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_8044_a2ab_f5e5d54af373_TB3245_11.jpg) . [Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]
. [Atomic masses: thorium-230 = 230.033127 amu; helium-4 = 4.002603 amu; radium-226 = 226.025403 amu]A)3.98 109 kJ/mol
B)4.60 108 kJ/mol
C)7.20 1011 kJ/mol
D)4.90 109 kJ/mol
E)7.15 1011 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
23
The 14C activity of some ancient Peruvian corn was found to be 10 disintegrations per minute per gram of carbon. If present-day plant life shows 15 dpm/g, how old is the Peruvian corn? The half-life of 14C is 5730 yr.
A)1,455 yr
B)1,910 yr
C)3,350 yr
D)3,820 yr
E)9,080 yr
A)1,455 yr
B)1,910 yr
C)3,350 yr
D)3,820 yr
E)9,080 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
24
A rock contains 0.37 mg of Pb-206 and 0.95 mg of U-238. The half-life of the decay series U-238 Pb-206 is 4.5 109 yr. Assuming no Pb-206 was present in the rock initially, how old is the rock?
A)1.7 109 yr
B)5.2 109 yr
C)2.7 106 yr
D)4.5 109 yr
E)2.4 109 yr
A)1.7 109 yr
B)5.2 109 yr
C)2.7 106 yr
D)4.5 109 yr
E)2.4 109 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
25
Calculate the energy released in joules when one mole of polonium-214 decays according to the equation ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5930_a2ab_a1adf0522399_TB3245_11.jpg) Po
Po ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5931_a2ab_777b13867394_TB3245_11.jpg)
![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5932_a2ab_151168fee9cb_TB3245_11.jpg) Pb +
Pb + ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5933_a2ab_53dc12db98ec_TB3245_11.jpg) He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
A)8.78 1014 J/mol
B)7.2 1014 J/mol
C)8.78 1011 J/mol
D)-9.75 10-3 J/mol
E)1.46 10-9 J/mol
![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5930_a2ab_a1adf0522399_TB3245_11.jpg) Po
Po ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5931_a2ab_777b13867394_TB3245_11.jpg)
![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5932_a2ab_151168fee9cb_TB3245_11.jpg) Pb +
Pb + ![<strong>Calculate the energy released in joules when one mole of polonium-214 decays according to the equation Po Pb + He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]</strong> A)8.78 \times 10<sup>14</sup> J/mol B)7.2 \times 10<sup>14</sup> J/mol C)8.78 \times 10<sup>11</sup> J/mol D)-9.75 \times 10<sup>-3</sup> J/mol E)1.46 \times 10<sup>-9</sup> J/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_5933_a2ab_53dc12db98ec_TB3245_11.jpg) He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]
He. [Atomic masses: Pb-210 = 209.98284 amu, Po-214 = 213.99519 amu, He-4 = 4.00260 amu.]A)8.78 1014 J/mol
B)7.2 1014 J/mol
C)8.78 1011 J/mol
D)-9.75 10-3 J/mol
E)1.46 10-9 J/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
26
The heaviest known isotope of hydrogen is called tritium, 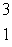 H. It decays by beta emission, and has a half-life of 12.3 years. What fraction of a tritium sample will remain after 5.20 years?
H. It decays by beta emission, and has a half-life of 12.3 years. What fraction of a tritium sample will remain after 5.20 years?
A)0.0210
B)0.746
C)3.41
D)0.254
E)0.423
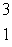 H. It decays by beta emission, and has a half-life of 12.3 years. What fraction of a tritium sample will remain after 5.20 years?
H. It decays by beta emission, and has a half-life of 12.3 years. What fraction of a tritium sample will remain after 5.20 years?A)0.0210
B)0.746
C)3.41
D)0.254
E)0.423

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
27
Cobalt-60 is a beta emitter with a half-life of 5.3 years. Approximately what fraction of the cobalt-60 atoms in a particular sample will remain after 32 years?
A)1/6
B)1/8
C)1/16
D)1/32
E)1/64
A)1/6
B)1/8
C)1/16
D)1/32
E)1/64

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
28
If 12% of a certain radioisotope decays in 5.2 years, what is the half-life of this isotope?
A)0.59 yr
B)1.7 yr
C)22 yr
D)28 yr
E)32 yr
A)0.59 yr
B)1.7 yr
C)22 yr
D)28 yr
E)32 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
29
The only stable isotope of fluorine is 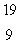 F. What type of radioactivity would you expect from the isotope
F. What type of radioactivity would you expect from the isotope 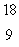 F, which has one fewer neutron?
F, which has one fewer neutron?
A)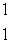 p
p
B)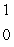 n
n
C)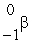
D)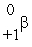
E)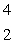 He
He
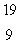 F. What type of radioactivity would you expect from the isotope
F. What type of radioactivity would you expect from the isotope 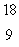 F, which has one fewer neutron?
F, which has one fewer neutron?A)
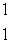 p
pB)
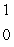 n
nC)
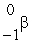
D)
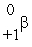
E)
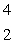 He
He
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
30
Polonium-208 is an alpha emitter with a half-life of 2.90 years. How many milligrams of polonium from an original sample of 2.00 mg will remain after 8.00 years?
A)0.147 mg
B)0.296 mg
C)0.725 mg
D)6.77 mg
E)1.90 mg
A)0.147 mg
B)0.296 mg
C)0.725 mg
D)6.77 mg
E)1.90 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
31
Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine.Tritium decays by beta decay and has a half-life of 12.3 yr. 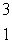 H
H 
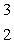 He +
He + 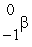
A)0.029 yr
B)7.4 yr
C)9.1 yr
D)16 yr
E)35 yr
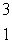 H
H 
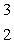 He +
He + 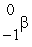
A)0.029 yr
B)7.4 yr
C)9.1 yr
D)16 yr
E)35 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
32
Determine how much energy is released when polonium-210 decays according to ![<strong>Determine how much energy is released when polonium-210 decays according to . [Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]</strong> A)4.14 \times 10<sup>9</sup> kJ/mol B)7.20 \times 10<sup>11</sup> kJ/mol C)5.22 \times 10<sup>8</sup> kJ/mol D)4.66 \times 10<sup>9</sup> kJ/mol E)6.43 \times 10<sup>12</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_8045_a2ab_f5090605275f_TB3245_11.jpg) . [Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]
. [Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]
A)4.14 109 kJ/mol
B)7.20 1011 kJ/mol
C)5.22 108 kJ/mol
D)4.66 109 kJ/mol
E)6.43 1012 kJ/mol
![<strong>Determine how much energy is released when polonium-210 decays according to . [Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]</strong> A)4.14 \times 10<sup>9</sup> kJ/mol B)7.20 \times 10<sup>11</sup> kJ/mol C)5.22 \times 10<sup>8</sup> kJ/mol D)4.66 \times 10<sup>9</sup> kJ/mol E)6.43 \times 10<sup>12</sup> kJ/mol](https://d2lvgg3v3hfg70.cloudfront.net/TB3245/11ea7cc5_01d1_8045_a2ab_f5090605275f_TB3245_11.jpg) . [Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]
. [Atomic masses: polonium-210 = 209.982857 amu; helium-4 = 4.002603 amu; lead-206 = 205.974449 amu]A)4.14 109 kJ/mol
B)7.20 1011 kJ/mol
C)5.22 108 kJ/mol
D)4.66 109 kJ/mol
E)6.43 1012 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
33
How old is a bottle of wine if the tritium (3H)content is 25% that of a new wine? The half-life of tritium is 12.5 years.
A)0.25 yr
B)3.1 yr
C)25 yr
D)38 yr
E)50.yr
A)0.25 yr
B)3.1 yr
C)25 yr
D)38 yr
E)50.yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
34
Charcoal samples taken from holes dug at Stonehenge, England, have a carbon-14 specific activity of 9.50 dpm per gram carbon. Living wood has a specific activity of 15.3 dpm per gram of carbon.Given that the half-life of carbon-14 is 5730 yr, how long ago was the wood part of a living plant?
A)3940 yr
B)3550 yr
C)9230 yr
D)5700 yr
E)13300 yr
A)3940 yr
B)3550 yr
C)9230 yr
D)5700 yr
E)13300 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
35
Charcoal found under a stone at Stonehenge, England, has a carbon-14 activity that is 0.60 that of new wood.How old is the charcoal? (The half-life of carbon-14 is 5,730 years.)
A)Less than 5,730 yr
B)Between 5,730 and 11,460 yr
C)Between 11,460 and 17,190 yr
D)More than 17,190 yr
A)Less than 5,730 yr
B)Between 5,730 and 11,460 yr
C)Between 11,460 and 17,190 yr
D)More than 17,190 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
36
What fraction of radioactive atoms remains in a sample after six half-lives?
A)zero
B)1/6
C)1/16
D)1/32
E)1/64
A)zero
B)1/6
C)1/16
D)1/32
E)1/64

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
37
Carbon-11 is a radioactive isotope of carbon. Its half-life is 20.3 minutes. What fraction of the initial number of carbon-11 atoms in a sample will remain after 81 minutes?
A)1/16
B)1/4
C)1/2
D)1/32
E)1/8
A)1/16
B)1/4
C)1/2
D)1/32
E)1/8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
38
Cobalt-60 is a beta emitter with a half-life of 5.3 years. Approximately what fraction of cobalt-60 atoms will remain in a particular sample after 26.5 years?
A)1/5
B)1/16
C)1/26
D)1/32
E)1/64
A)1/5
B)1/16
C)1/26
D)1/32
E)1/64

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
39
Find the nuclear binding enrgy of uranium-234 (atomic mass = 234.040947 amu)in units of joules per nucleon.[Data: neutron mass = 1.674928 10-24 g; proton mass = 1.672623 10-24 g; electron mass = 9.109387 10-28 g; NA = 6.0221367 1023 /mol; c = 2.99792458 108 m/s]
A)2.97 10-10 J/nucleon
B)3.04 10-10 J/nucleon
C)1.30 10-12 J/nucleon
D)1.22 10-12 J/nucleon
E)1.27 10-12 J/nucleon
A)2.97 10-10 J/nucleon
B)3.04 10-10 J/nucleon
C)1.30 10-12 J/nucleon
D)1.22 10-12 J/nucleon
E)1.27 10-12 J/nucleon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
40
The half-life of 90Sr is 29 years. What fraction of the atoms in a sample of 90Sr would remain 175 years later?
A)0.17
B)0.12
C)0.062
D)0.015
E)0.50
A)0.17
B)0.12
C)0.062
D)0.015
E)0.50

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
41
The half-life of 14C is 5,730 yr. Assuming some charcoal from a campfire 29,000 years old was found, what fraction of the original C-14 would remain today?
A)3.0 10-2
B)0.197
C)3.51
D)33.3
E)None of these.
A)3.0 10-2
B)0.197
C)3.51
D)33.3
E)None of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
42
In passing through matter, alpha particles lose energy chiefly by causing
A)fermentation.
B)neutralization.
C)ionization.
D)condensation.
E)carbonation.
A)fermentation.
B)neutralization.
C)ionization.
D)condensation.
E)carbonation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which is the most penetrating of the three types of nuclear radiation?
A)alpha
B)beta
C)gamma
A)alpha
B)beta
C)gamma

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
44
Petroleum is a fossil fuel containing many different carbon compounds. If the carbon atoms in petroleum have been in the ground for 100 million years, what fraction of the initial 14C atoms is still there? (t1/2 = 5,370 yr)
A)0
B)1 10-10
C)5.7 10-5
D)1.0 10-3
E)5.7 10-1
A)0
B)1 10-10
C)5.7 10-5
D)1.0 10-3
E)5.7 10-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
45
Rubidium-87 decays by beta decay with a half-life of 4.9 1010 yr. How many 87Rb atoms are in a moon rock sample that has a rubidium decay rate of 3,500 disintegrations per hour?
A)9.0 1016 atoms
B)4.3 10-4 atoms
C)2.2 1018 atoms
D)2.5 1014 atoms
E)1.7 1014 atoms
A)9.0 1016 atoms
B)4.3 10-4 atoms
C)2.2 1018 atoms
D)2.5 1014 atoms
E)1.7 1014 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
46
Gamma-rays cause radiation damage when they interact with matter by producing
A)ions and free radicals.
B)isotopes.
C)daughter products.
D)oxidation.
E)reduction.
A)ions and free radicals.
B)isotopes.
C)daughter products.
D)oxidation.
E)reduction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
47
What element is the stable end-product of the uranium radioactive decay series?
A)Th
B)Pu
C)Ra
D)Au
E)Pb
A)Th
B)Pu
C)Ra
D)Au
E)Pb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
48
The Rb-87/Sr-87 method of dating rocks is often used by geologists: 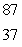 Rb
Rb 
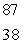 Sr +
Sr + 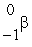 t1/2 = 6.0 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.
t1/2 = 6.0 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.
A)2.4 109 yr
B)1.7 109 yr
C)3.1 1011 yr
D)4.1 10-11 yr
E)3.6 1011 yr
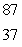 Rb
Rb 
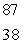 Sr +
Sr + 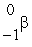 t1/2 = 6.0 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.
t1/2 = 6.0 1010 yr Estimate the age of a rock sample in which the present-day mole ratio of Rb-87 to Sr-87 is 36:1.A)2.4 109 yr
B)1.7 109 yr
C)3.1 1011 yr
D)4.1 10-11 yr
E)3.6 1011 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which type of nuclear process requires an extremely high temperature (millions of degrees)?
A)beta decay
B)fission reaction
C)fusion reaction
D)alpha decay
E)positron emission
A)beta decay
B)fission reaction
C)fusion reaction
D)alpha decay
E)positron emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which one of the following statements about fission and fusion is false?
A)Fission occurs among the heaviest isotopes, whereas fusion occurs more readily for light isotopes.
B)For a fission reaction the mass defect ( m)is negative, whereas for fusion m is positive.
C)In order for fusion reactions to occur, temperatures must be in the millions of degrees.
D)The fission of Pu-239 atoms produces a great number of isotopes of a large number of elements.
E)Neutron-induced fission processes can occur at room temperature, rather than at millions of degrees.
A)Fission occurs among the heaviest isotopes, whereas fusion occurs more readily for light isotopes.
B)For a fission reaction the mass defect ( m)is negative, whereas for fusion m is positive.
C)In order for fusion reactions to occur, temperatures must be in the millions of degrees.
D)The fission of Pu-239 atoms produces a great number of isotopes of a large number of elements.
E)Neutron-induced fission processes can occur at room temperature, rather than at millions of degrees.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
51
Present-day plant life has a carbon-14 decay rate of 16 disintegrations per minute (dpm)per gram of carbon.If a contemporary wooden chair were somehow preserved for the next 3,900 years, what 14C decay rate should be expected from the wood used to make the chair? (t1/2 = 5,730 yr)
A)26 dpm
B)12 dpm
C)11 dpm
D)10 dpm
E)8 dpm
A)26 dpm
B)12 dpm
C)11 dpm
D)10 dpm
E)8 dpm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
52
The energy released by the sun is the result of
A)natural radioactivity.
B)nuclear fusion.
C)combustion of hydrogen.
D)photosynthesis.
E)nuclear fission.
A)natural radioactivity.
B)nuclear fusion.
C)combustion of hydrogen.
D)photosynthesis.
E)nuclear fission.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
53
The carbon-14 activity of some ancient Indian corn was found to be 7.0 disintegrations per minute (dpm)per gram of carbon. If present-day plant life has 16 dpm per gram of carbon, how old is the Indian corn? (t1/2 = 5,730 yr)
A)6,800 yr
B)2,500 yr
C)4,700 yr
D)10,000 yr
E)7,200 yr
A)6,800 yr
B)2,500 yr
C)4,700 yr
D)10,000 yr
E)7,200 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
54
What role does cadmium metal (Cd)play in a nuclear reactor?
A)slows down the fission neutrons (moderator)
B)transfers heat from the reactor to the heat exchanger (primary coolant)
C)controls chain reaction (control rods)
D)transfers heat from the condenser to the environment (cooling tower)
E)undergoes fission (fuel rods)
A)slows down the fission neutrons (moderator)
B)transfers heat from the reactor to the heat exchanger (primary coolant)
C)controls chain reaction (control rods)
D)transfers heat from the condenser to the environment (cooling tower)
E)undergoes fission (fuel rods)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
55
The radioisotope potassium-40 decays to argon-40 by positron emission with a half-life of 1.3 109 yr. A sample of moon rock was found to contain 78 argon-40 atoms for every 22 potassium-40 atoms. The age of the rock is
A)8.1 10-10 yr
B)2.4 109 yr
C)2.8 109 yr
D)4.6 109 yr
E)6.8 109 yr
A)8.1 10-10 yr
B)2.4 109 yr
C)2.8 109 yr
D)4.6 109 yr
E)6.8 109 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
56
A sample of a radioisotope shows an activity of 999 disintegrations per minute due to beta decay. If after 1.10 years the activity is 952 disintegrations per minute, what is the half-life of this radioisotope?
A)4.38 10-2 yr
B)11.4 yr
C)0.25 yr
D)15.8 yr
E)9.1 yr
A)4.38 10-2 yr
B)11.4 yr
C)0.25 yr
D)15.8 yr
E)9.1 yr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
57
What would the atom ratio of 206Pb to 238U be in a uranium mineral from a rock that is 1.0 109 years old? t1/2(238U)= 4.5 109 yr.
A)0.14
B)0.16
C)0.22
D)0.86
E)1.16
A)0.14
B)0.16
C)0.22
D)0.86
E)1.16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which type of radiation is the least penetrating?
A)alpha
B)beta
C)gamma
A)alpha
B)beta
C)gamma

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following is an advantage of nuclear power plants over coal-burning plants? Nuclear power plants
A)produce radioactive byproducts with very short half-lives, reducing the need for waste storage.
B)do not pollute the air with SO2, soot, and fly-ash.
C)create no thermal pollution.
D)generate radioactive byproducts that can be sold for use in secondary applications.
A)produce radioactive byproducts with very short half-lives, reducing the need for waste storage.
B)do not pollute the air with SO2, soot, and fly-ash.
C)create no thermal pollution.
D)generate radioactive byproducts that can be sold for use in secondary applications.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
60
The dose unit of ionizing radiation is called the rad. The rad is defined in terms of
A)the half-life of a radioisotope.
B)the energy deposited per gram of an object.
C)the biological damage produced.
D)the accumulation of fission products.
E)the number of ions per centimeter.
A)the half-life of a radioisotope.
B)the energy deposited per gram of an object.
C)the biological damage produced.
D)the accumulation of fission products.
E)the number of ions per centimeter.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
61
Decay of silicon-27 by positron emission yields
A)magnesium-23.
B)sulfur-31.
C)phosphorus-27.
D)silicon-26.
E)aluminum-27.
A)magnesium-23.
B)sulfur-31.
C)phosphorus-27.
D)silicon-26.
E)aluminum-27.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
62
Decay of ruthenium-93 by positron emission yields
A)technetium-93.
B)molybdenum-89.
C)palladium-97.
D)rhenium-93.
E)ruthenium-92.
A)technetium-93.
B)molybdenum-89.
C)palladium-97.
D)rhenium-93.
E)ruthenium-92.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
63
Complete and balance the nuclear equation 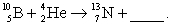
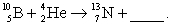

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
64
In the following reaction, identify X. 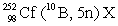
A)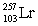
B)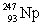
C)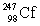
D)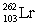
E)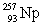
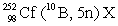
A)
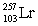
B)
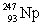
C)
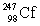
D)
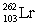
E)
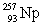

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
65
Complete and balance the nuclear equation 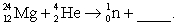
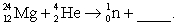

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which isotope, when bombarded with nitrogen-15, yields the artificial isotope dubnium-260 plus 4 neutrons?
A)californium-245
B)thorium-257
C)nobelium-245
D)californium-249
E)dubnium-249
A)californium-245
B)thorium-257
C)nobelium-245
D)californium-249
E)dubnium-249

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
67
Tritium is a radioisotope of hydrogen having a half-life of 12.3 years.If you initially had 1.0 10-7 mole of tritium, how many moles of tritium would be left after 78 years?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
68
Complete and balance the nuclear equation 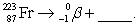
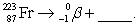

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
69
How many 14C atoms are in a charcoal sample that has a decay rate of 3,500 disintegrations per min? (For 14C, t1/2 = 5,730 yr.)
A)2.9 107 atoms
B)8.0 10-7 atoms
C)1.4 1014 atoms
D)1.5 1013 atoms
E)6.02 1020 atoms
A)2.9 107 atoms
B)8.0 10-7 atoms
C)1.4 1014 atoms
D)1.5 1013 atoms
E)6.02 1020 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
70
Decay of tin-110 by electron capture yields
A)tin-109.
B)cadmium-106.
C)indium-110.
D)antimony-110.
E)tellurium-114.
A)tin-109.
B)cadmium-106.
C)indium-110.
D)antimony-110.
E)tellurium-114.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
71
In the following reaction, identify X. 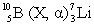
A)()
B)n
C)p
D) (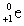 )
)
E)()
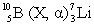
A)()
B)n
C)p
D) (
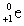 )
)E)()

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
72
Tritium is a radioisotope of hydrogen having a half-life of 12.3 years.If you initially had 1.0 10-7 mole of tritium, calculate the decay rate of the sample.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
73
In the following reaction, identify X. 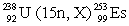
A)7
B)3
C)4n
D)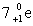
E)15p
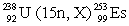
A)7
B)3
C)4n
D)
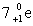
E)15p

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the decay rate of a sample of a radioisotope that has an activity of 12 microcuries? [1 curie = 3.7 1010 disintegrations/s]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
75
Complete and balance the nuclear equation 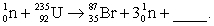
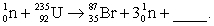

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
76
Decay of lutetium-167 by electron capture yields
A)ytterbium-167.
B)lutetium-166.
C)thulium-163.
D)tantalum-171.
E)hafnium-167.
A)ytterbium-167.
B)lutetium-166.
C)thulium-163.
D)tantalum-171.
E)hafnium-167.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
77
Complete and balance the nuclear equation 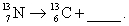
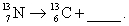

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which isotope, when bombarded with oxygen-18, yields the artificial isotope seaborgium-263 plus 4 neutrons?
A)nobelium-245
B)radium-259
C)californium-245
D)nobelium-249
E)californium-249
A)nobelium-245
B)radium-259
C)californium-245
D)nobelium-249
E)californium-249

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
79
On a neutron number versus proton number plot, the nucleus of oxygen-15 lies below the belt of stability. Write an equation for radioactive decay of oxygen-15.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
80
On a plot of the number of neutrons versus the number of protons, a nucleus of aluminum-28 lies above the belt of stability. Write an equation for radioactive decay of aluminum-28.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck








