Deck 6: Electronic Structure of Atoms
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/186
العب
ملء الشاشة (f)
Deck 6: Electronic Structure of Atoms
1
All of the orbitals in a given electron shell have the same value as the ________ quantum number.
A)principal
B)angular momentum
C)magnetic
D)spin
E)psi
A)principal
B)angular momentum
C)magnetic
D)spin
E)psi
principal
2
Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number?
A)4f
B)4d
C)4p
D)4s
E)none of the above
A)4f
B)4d
C)4p
D)4s
E)none of the above
none of the above
3
All of the orbitals in a given subshell have the same value as the ________ quantum number.
A)principal
B)angular momentum
C)magnetic
D)A and B
E)B and C
A)principal
B)angular momentum
C)magnetic
D)A and B
E)B and C
A and B
4
Which one of the following is correct?
A)ν + λ = c
B)ν ÷ λ = c
C)ν = cλ
D)λ = c ν
E)νλ = c
A)ν + λ = c
B)ν ÷ λ = c
C)ν = cλ
D)λ = c ν
E)νλ = c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
5
Of the following transitions in the Bohr hydrogen atom, the ________ transition results in the emission of the lowest-energy photon.
A)n = 1 → n = 6
B)n = 6 → n = 1
C)n = 6 → n = 3
D)n = 3 → n = 6
E)n = 1 → n = 4
A)n = 1 → n = 6
B)n = 6 → n = 1
C)n = 6 → n = 3
D)n = 3 → n = 6
E)n = 1 → n = 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which one of the following is an incorrect orbital notation?
A)2s
B)3py
C)3f
D)4dxy
E)4s
A)2s
B)3py
C)3f
D)4dxy
E)4s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which one of the following is considered to be ionizing radiation?
A)visible light
B)radio waves
C)X-rays
D)microwaves
E)infrared radiation
A)visible light
B)radio waves
C)X-rays
D)microwaves
E)infrared radiation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which one of the following is not a valid value for the magnetic quantum number of an electron in a 5d subshell?
A)2
B)3
C)0
D)1
E)-1
A)2
B)3
C)0
D)1
E)-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
9
An electron cannot have the quantum numbers n = ________, l = ________, ml = ________.
A)6, 1, 0
B)3, 2, 3
C)3, 2, -2
D)1, 0, 0
E)3, 2, 1
A)6, 1, 0
B)3, 2, 3
C)3, 2, -2
D)1, 0, 0
E)3, 2, 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
10
An electron cannot have the quantum numbers n = ________, l = ________, ml = ________.
A)2, 0, 0
B)2, 1, -1
C)3, 1, -1
D)1, 1, 1
E)3, 2, 1
A)2, 0, 0
B)2, 1, -1
C)3, 1, -1
D)1, 1, 1
E)3, 2, 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which one of the following is an incorrect subshell notation?
A)4f
B)2d
C)3s
D)2p
E)3d
A)4f
B)2d
C)3s
D)2p
E)3d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
12
The uncertainty principle states that ________.
A)matter and energy are really the same thing
B)it is impossible to know anything with certainty
C)it is impossible to know the exact position and momentum of an electron
D)there can only be one uncertain digit in a reported number
E)it is impossible to know how many electrons there are in an atom
A)matter and energy are really the same thing
B)it is impossible to know anything with certainty
C)it is impossible to know the exact position and momentum of an electron
D)there can only be one uncertain digit in a reported number
E)it is impossible to know how many electrons there are in an atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which quantum number determines the energy of an electron in a hydrogen atom?
A)n
B)E
C)ml
D)l
E)n and l
A)n
B)E
C)ml
D)l
E)n and l

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number?
A)2d
B)2s
C)2p
D)all of the above
E)none of the above
A)2d
B)2s
C)2p
D)all of the above
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
15
According to the Heisenberg Uncertainty Principle, it is impossible to know precisely both the position and the ________ of an electron.
A)mass
B)color
C)momentum
D)shape
E)charge
A)mass
B)color
C)momentum
D)shape
E)charge

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which one of the quantum numbers does not result from the solution of the Schrodinger equation?
A)principal
B)azimuthal
C)magnetic
D)spin
E)angular momentum
A)principal
B)azimuthal
C)magnetic
D)spin
E)angular momentum

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
17
The de Broglie wavelength of a ________ will have the shortest wavelength when traveling at 30 cm/s.
A)marble
B)car
C)planet
D)uranium atom
E)hydrogen atom
A)marble
B)car
C)planet
D)uranium atom
E)hydrogen atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
18
In the Bohr model of the atom, ________.
A)electrons travel in circular paths called orbitals
B)electrons can have any energy
C)electron energies are quantized
D)electron paths are controlled by probability
E)both A and C
A)electrons travel in circular paths called orbitals
B)electrons can have any energy
C)electron energies are quantized
D)electron paths are controlled by probability
E)both A and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
19
Of the following, ________ radiation has the shortest wavelength.
A)X-ray
B)radio
C)microwave
D)ultraviolet
E)infrared
A)X-ray
B)radio
C)microwave
D)ultraviolet
E)infrared

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
20
The photoelectric effect is ________.
A)the total reflection of light by metals giving them their typical luster
B)the production of current by silicon solar cells when exposed to sunlight
C)the ejection of electrons by a metal when struck with light of sufficient energy
D)the darkening of photographic film when exposed to an electric field
E)a relativistic effect
A)the total reflection of light by metals giving them their typical luster
B)the production of current by silicon solar cells when exposed to sunlight
C)the ejection of electrons by a metal when struck with light of sufficient energy
D)the darkening of photographic film when exposed to an electric field
E)a relativistic effect

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which electron configuration denotes an atom in its ground state?
A)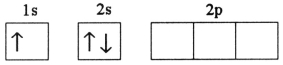
B)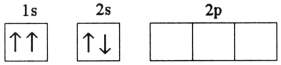
C)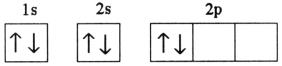
D)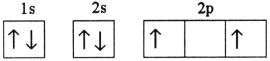
E)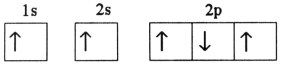
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which electron configuration represents a violation of the Pauli exclusion principle?
A)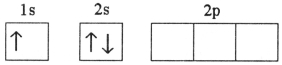
B)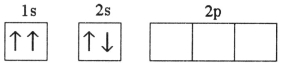
C)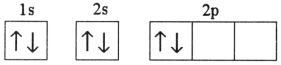
D)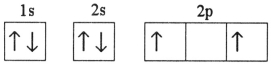
E)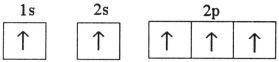
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
23
The ground-state electron configuration of the element ________ is [Kr]5s14d5.
A)Nb
B)Mo
C)Cr
D)Mn
E)Tc
A)Nb
B)Mo
C)Cr
D)Mn
E)Tc

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following is a valid set of four quantum numbers? (n, l, ml, ms)
A)2, 1, 0, +1/2
B)2, 2, 1, -1/2
C)1, 0, 1, +1/2
D)2, 1, +2, +1/2
E)1, 1, 0, -1/2
A)2, 1, 0, +1/2
B)2, 2, 1, -1/2
C)1, 0, 1, +1/2
D)2, 1, +2, +1/2
E)1, 1, 0, -1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which electron configuration represents a violation of Hund's rule for an atom in its ground state?
A)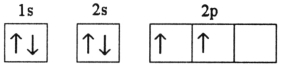
B)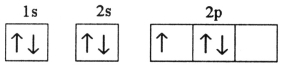
C)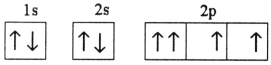
D)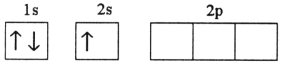
E)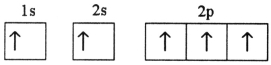
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
26
In a px orbital, the subscript x denotes the ________.
A)energy of the electron
B)spin of the electrons
C)probability of the shell
D)size of the orbital
E)axis along which the orbital is aligned
A)energy of the electron
B)spin of the electrons
C)probability of the shell
D)size of the orbital
E)axis along which the orbital is aligned

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which quantum numbers must be the same for the orbitals that they designate to be degenerate in a one-electron system (such as hydrogen)?
A)n, l, and ml
B)n and l only
C)l and ml
D)ml only
E)n only
A)n, l, and ml
B)n and l only
C)l and ml
D)ml only
E)n only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following is not a valid set of four quantum numbers? (n, l, ml, ms)
A)2, 0, 0, +1/2
B)2, 1, 0, -1/2
C)3, 1, -1, -1/2
D)1, 0, 0, +1/2
E)1, 1, 0, +1/2
A)2, 0, 0, +1/2
B)2, 1, 0, -1/2
C)3, 1, -1, -1/2
D)1, 0, 0, +1/2
E)1, 1, 0, +1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which electron configuration represents a violation of Hund's rule for an atom in its ground state?
A)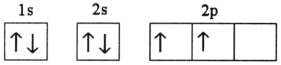
B)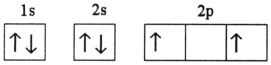
C)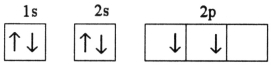
D)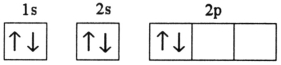
E)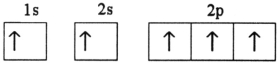
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which one of the following is the correct electron configuration for a ground-state nitrogen atom?
A)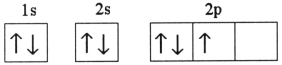
B)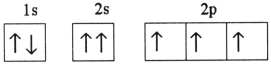
C)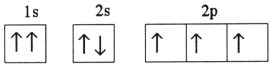
D)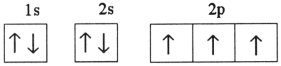
E)None of the above is correct.
A)

B)

C)

D)

E)None of the above is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which electron configuration represents a violation of the Pauli exclusion principle?
A)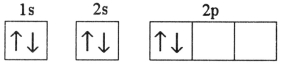
B)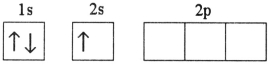
C)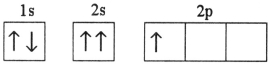
D)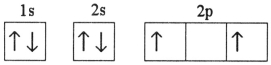
E)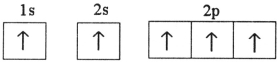
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
32
The ground-state electron configuration of ________ is [Ar]4s13d5.
A)V
B)Mn
C)Fe
D)Cr
E)K
A)V
B)Mn
C)Fe
D)Cr
E)K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
33
The ground state electron configuration of Ga is ________.
A)1s22s23s23p64s23d104p1
B)1s22s22p63s23p64s24d104p1
C)1s22s22p63s23p64s23d104p1
D)1s22s22p63s23p64s23d104d1
E)[Ar]4s23d11
A)1s22s23s23p64s23d104p1
B)1s22s22p63s23p64s24d104p1
C)1s22s22p63s23p64s23d104p1
D)1s22s22p63s23p64s23d104d1
E)[Ar]4s23d11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which one of the following configurations depicts an excited oxygen atom?
A)1s22s22p2
B)1s22s22p23s2
C)1s22s22p1
D)1s22s22p4
E)[He]2s22p4
A)1s22s22p2
B)1s22s22p23s2
C)1s22s22p1
D)1s22s22p4
E)[He]2s22p4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
35
The ________ orbital is degenerate with 5py in a many-electron atom.
A)5s
B)5px
C)4py
D)5dxy
E)5d2
A)5s
B)5px
C)4py
D)5dxy
E)5d2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which one of the following orbitals can hold two electrons?
A)2px
B)3s
C)4dxy
D)all of the above
E)none of the above
A)2px
B)3s
C)4dxy
D)all of the above
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which electron configuration represents a violation of Hund's rule for an atom in its ground state?
A)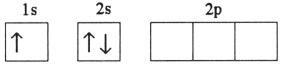
B)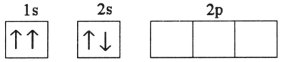
C)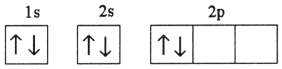
D)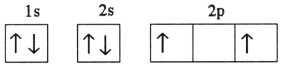
E)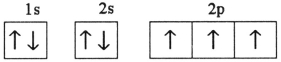
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which electron configuration represents a violation of the Pauli exclusion principle?
A)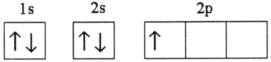
B)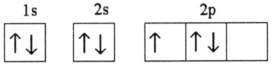
C)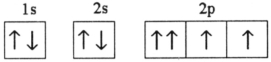
D)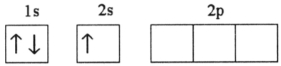
E)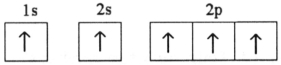
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which quantum numbers must be the same for the orbitals that they designate to be degenerate in a many-electron system?
A)n, l, and ml
B)n only
C)n, l, ml, and ms
D)ms only
E)n and l only
A)n, l, and ml
B)n only
C)n, l, ml, and ms
D)ms only
E)n and l only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which one of the following configurations depicts an excited carbon atom?
A)1s22s22p13s1
B)1s22s22p3
C)1s22s22p1
D)1s22s23s1
E)1s22s22p2
A)1s22s22p13s1
B)1s22s22p3
C)1s22s22p1
D)1s22s23s1
E)1s22s22p2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
41
The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes ________.
A)Pauli Exclusion Principle
B)Planck's constant
C)deBroglie hypothesis
D)Heisenberg Uncertainty Principle
E)Hund's rule
A)Pauli Exclusion Principle
B)Planck's constant
C)deBroglie hypothesis
D)Heisenberg Uncertainty Principle
E)Hund's rule

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
42
The ground-state configuration of fluorine is ________.
A)[He]2s22p2
B)[He]2s22p3
C)[He]2s22p4
D)[He]22s22p5
E)[He]2s22p6
A)[He]2s22p2
B)[He]2s22p3
C)[He]2s22p4
D)[He]22s22p5
E)[He]2s22p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
43
Ham radio operators often broadcast on the 6-meter band. The frequency of this electromagnetic radiation is ________ MHz.
A)500
B)200
C)50
D)20
E)2.0
A)500
B)200
C)50
D)20
E)2.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
44
The ground-state configuration of tungsten is ________.
A)[Ar]4s23d3
B)[Xe]6s24f145d4
C)[Ne]3s1
D)[Xe]6s24f7
E)[Kr]5s24d105p5
A)[Ar]4s23d3
B)[Xe]6s24f145d4
C)[Ne]3s1
D)[Xe]6s24f7
E)[Kr]5s24d105p5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the frequency of light (s-1)that has a wavelength of 3.12 × 10-3 cm?
A)3.69 s-1
B)2.44 × 1016 s-1
C)9.62 × 1012 s-1
D)4.10 × 10-17 s-1
E)1.04 × 10-13 s-1
A)3.69 s-1
B)2.44 × 1016 s-1
C)9.62 × 1012 s-1
D)4.10 × 10-17 s-1
E)1.04 × 10-13 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the frequency of light (s-1)that has a wavelength of 1.23 × 10-6 cm?
A)3.69
B)2.44 × 1016 s-1
C)4.10 × 10-17 s-1
D)9.62 × 1012 s-1
E)1.04 × 10-13 s-1
A)3.69
B)2.44 × 1016 s-1
C)4.10 × 10-17 s-1
D)9.62 × 1012 s-1
E)1.04 × 10-13 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
47
How many different principal quantum numbers can be found in the ground-state electron configuration of nickel?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
48
The wavelength of light that has a frequency of 1.66 × 109 s-1 is ________ m.
A)0.181
B)5.53
C)2.00 × 10-9
D)5.53 × 108
E)none of the above
A)0.181
B)5.53
C)2.00 × 10-9
D)5.53 × 108
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
49
The valence shell of the element X contains 2 electrons in a 5s subshell. Below that shell, element X has a partially filled 4d subshell. What type of element is X?
A)main group element
B)chalcogen
C)halogen
D)transition metal
E)alkali metal
A)main group element
B)chalcogen
C)halogen
D)transition metal
E)alkali metal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the frequency (s-1)of electromagnetic radiation that has a wavelength of 2.3 m?
A)1.3 × 108 s-1
B)1.8 × 10-9 s-1
C)1.6 × 108 s-1
D)1.3 × 10-33 s-1
E)1.3 × 1033 s-1
A)1.3 × 108 s-1
B)1.8 × 10-9 s-1
C)1.6 × 108 s-1
D)1.3 × 10-33 s-1
E)1.3 × 1033 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
51
Electromagnetic radiation travels through vacuum at a speed of ________ m/s.
A)186,000
B)125
C)3.00 × 108
D)10,000
E)It depends on wavelength.
A)186,000
B)125
C)3.00 × 108
D)10,000
E)It depends on wavelength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following elements has a ground-state electron configuration different from the predicted one?
A)Cu
B)Ca
C)Xe
D)Cl
E)Ti
A)Cu
B)Ca
C)Xe
D)Cl
E)Ti

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
53
The wavelength of light that has a frequency of 1.20 × 1013 s-1 is ________ m.
A)25.0
B)2.50 × 10-5
C)0.0400
D)12.0
E)2.5
A)25.0
B)2.50 × 10-5
C)0.0400
D)12.0
E)2.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which two elements have the same ground-state electron configuration?
A)Pd and Pt
B)Cu and Ag
C)Fe and Cu
D)Cl and Ar
E)No two elements have the same ground-state electron configuration.
A)Pd and Pt
B)Cu and Ag
C)Fe and Cu
D)Cl and Ar
E)No two elements have the same ground-state electron configuration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
55
What is the frequency of light (s-1)that has a wavelength of 3.86 × 10-5 cm?
A)7.77 × 1014 s-1
B)6.32 × 10-12 s-1
C)1.04 × 10-13 s-1
D)9.62 × 1012 s-1
E)2.14 × 10-16 s-1
A)7.77 × 1014 s-1
B)6.32 × 10-12 s-1
C)1.04 × 10-13 s-1
D)9.62 × 1012 s-1
E)2.14 × 10-16 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the wavelength of light (nm)that has a frequency of 6.44 × 1013 s-1?
A)4660 nm
B)6490 nm
C)4.66 × 10-8 nm
D)6.49 × 10-8 nm
E)932 nm
A)4660 nm
B)6490 nm
C)4.66 × 10-8 nm
D)6.49 × 10-8 nm
E)932 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
57
What is the frequency (s-1)of electromagnetic radiation that has a wavelength of 0.53 m?
A)5.7 × 108 s-1
B)1.8 × 10-9 s-1
C)1.6 × 108 s-1
D)1.3 × 10-33 s-1
E)1.3 × 1033 s-1
A)5.7 × 108 s-1
B)1.8 × 10-9 s-1
C)1.6 × 108 s-1
D)1.3 × 10-33 s-1
E)1.3 × 1033 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
58
The wavelength of a photon that has an energy of 6.33 × 10-18 J is ________ m.
A)3.79 × 10-7
B)3.10 × 10-8
C)2.38 × 1023
D)4.21 × 10-24
E)9.55 × 1015
A)3.79 × 10-7
B)3.10 × 10-8
C)2.38 × 1023
D)4.21 × 10-24
E)9.55 × 1015

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
59
What is the wavelength of light (nm)that has a frequency 4.62 × 1014 s-1?
A)932 nm
B)649 nm
C)1.39 × 1023 nm
D)1.54 × 10-3 nm
E)1.07 × 106 nm
A)932 nm
B)649 nm
C)1.39 × 1023 nm
D)1.54 × 10-3 nm
E)1.07 × 106 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the wavelength of light (nm)that has a frequency of 3.22 × 1014 s-1?
A)932 nm
B)649 nm
C)9.66 × 1022 nm
D)9.32 × 10-7 nm
E)1.07 × 106 nm
A)932 nm
B)649 nm
C)9.66 × 1022 nm
D)9.32 × 10-7 nm
E)1.07 × 106 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
61
Of the following, ________ radiation has the longest wavelength and ________ radiation has the greatest energy. gamma ultraviolet visible
A)ultraviolet, gamma
B)visible, ultraviolet
C)gamma, gamma
D)visible, gamma
E)gamma, visible
A)ultraviolet, gamma
B)visible, ultraviolet
C)gamma, gamma
D)visible, gamma
E)gamma, visible

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
62
The energy of a photon that has a wavelength of 8.33 × 10-6 m is ________ J.
A)2.20 × 10-26
B)3.60 × 1013
C)2.39 × 10-20
D)2.7 × 109
E)4.5 × 10-25
A)2.20 × 10-26
B)3.60 × 1013
C)2.39 × 10-20
D)2.7 × 109
E)4.5 × 10-25

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
63
The energy of a photon that has a wavelength of 9.0 m is ________ J.
A)2.2 × 10-26
B)4.5 × 1025
C)6.0 × 10-23
D)2.7 × 109
E)4.5 × 10-25
A)2.2 × 10-26
B)4.5 × 1025
C)6.0 × 10-23
D)2.7 × 109
E)4.5 × 10-25

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
64
A mole of yellow photons of wavelength 527 nm has ________ kJ of energy.
A)165
B)227
C)4.56 × 10-46
D)6.05 × 10-3
E)2.74 × 10-19
A)165
B)227
C)4.56 × 10-46
D)6.05 × 10-3
E)2.74 × 10-19

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
65
A mole of red photons of wavelength 725 nm has ________ kJ of energy.
A)2.74 × 10-19
B)4.56 × 10-46
C)6.05 × 10-3
D)165
E)227
A)2.74 × 10-19
B)4.56 × 10-46
C)6.05 × 10-3
D)165
E)227

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
66
The energy of a photon that has a frequency of 1.821 × 1016 s-1 is ________ J.
A)5.44 × 10-18
B)1.99 × 10-25
C)3.49 × 10-48
D)1.21 × 10-17
E)5.46 × 10-24
A)5.44 × 10-18
B)1.99 × 10-25
C)3.49 × 10-48
D)1.21 × 10-17
E)5.46 × 10-24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
67
Of the following, ________ radiation has the shortest wavelength and ________ radiation has the greatest energy. gamma ultraviolet visible
A)gamma, visible
B)visible, gamma
C)visible, ultraviolet
D)ultraviolet, gamma
E)gamma, gamma
A)gamma, visible
B)visible, gamma
C)visible, ultraviolet
D)ultraviolet, gamma
E)gamma, gamma

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the frequency (s-1)of a photon that has an energy of 4.38 × 10-18 J?
A)436 s-1
B)6.61 × 1015 s-1
C)1.45 × 10-16 s-1
D)2.30 ×107 s-1
E)1.31 × 10-9 s-1
A)436 s-1
B)6.61 × 1015 s-1
C)1.45 × 10-16 s-1
D)2.30 ×107 s-1
E)1.31 × 10-9 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
69
What color of visible light has the highest energy?
A)violet
B)blue
C)red
D)green
E)yellow
A)violet
B)blue
C)red
D)green
E)yellow

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
70
The energy of a photon that has a frequency of 8.21 × 1015 s-1 is ________ J.
A)8.08 × 10-50
B)1.99 × 10-25
C)5.44 × 10-18
D)1.24 × 1049
E)1.26 × 10-19
A)8.08 × 10-50
B)1.99 × 10-25
C)5.44 × 10-18
D)1.24 × 1049
E)1.26 × 10-19

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
71
The frequency of a photon that has an energy of 8.5 × 10-12 J is ________ s-1.
A)1.3 × 1022
B)1.8 × 10-16
C)2.5 × 10-15
D)5.4 × 10-8
E)2.5 × 1015
A)1.3 × 1022
B)1.8 × 10-16
C)2.5 × 10-15
D)5.4 × 10-8
E)2.5 × 1015

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
72
What color of visible light has the longest wavelength?
A)blue
B)violet
C)red
D)yellow
E)green
A)blue
B)violet
C)red
D)yellow
E)green

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
73
The energy of a photon of light is ________ proportional to its frequency and ________ proportional to its wavelength.
A)directly, directly
B)inversely, inversely
C)inversely, directly
D)directly, inversely
E)indirectly, not
A)directly, directly
B)inversely, inversely
C)inversely, directly
D)directly, inversely
E)indirectly, not

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
74
The wavelength of a photon that has an energy of 5.25 × 10-19 J is ________ m.
A)3.79 × 10-7
B)2.64 × 106
C)2.38 × 1023
D)4.21 × 10-24
E)3.79 × 107
A)3.79 × 10-7
B)2.64 × 106
C)2.38 × 1023
D)4.21 × 10-24
E)3.79 × 107

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
75
Using Bohr's equation for the energy levels of the electron in the hydrogen atom, determine the energy (J)of an electron in the n = 4 level.
A)-1.36 × 10-19
B)-5.45 × 10-19
C)-7.34 × 1018
D)-1.84 × 10-29
E)+1.84 × 10-29
A)-1.36 × 10-19
B)-5.45 × 10-19
C)-7.34 × 1018
D)-1.84 × 10-29
E)+1.84 × 10-29

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
76
The energy of a photon that has a wavelength of 12.3 nm is ________ J.
A)1.51 × 10-17
B)4.42 × 10-23
C)1.99 × 10-25
D)2.72 × 10-50
E)1.62 × 10-17
A)1.51 × 10-17
B)4.42 × 10-23
C)1.99 × 10-25
D)2.72 × 10-50
E)1.62 × 10-17

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
77
What is the wavelength (angstroms)of a photon that has an energy of 5.69 × 10-17 J?
A)454 angstroms
B)34.9 angstroms
C)6.89 × 1015 angstroms
D)3.66 × 109 angstroms
E)3.50 × 10-9 angstroms
A)454 angstroms
B)34.9 angstroms
C)6.89 × 1015 angstroms
D)3.66 × 109 angstroms
E)3.50 × 10-9 angstroms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
78
The energy of a photon that has a wavelength of 13.2 nm is ________ J.
A)9.55 × 10-25
B)1.62 × 10-17
C)1.99 × 10-25
D)4.42 × 10-23
E)1.51 × 10-17
A)9.55 × 10-25
B)1.62 × 10-17
C)1.99 × 10-25
D)4.42 × 10-23
E)1.51 × 10-17

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the wavelength (angstroms)of a photon that has an energy of 4.38 × 10-18 J?
A)45.4 angstroms
B)2.30 × 107 angstroms
C)6.89 × 1015 angstroms
D)1.45 × 10-16 angstroms
E)1.31 × 10-9 angstroms
A)45.4 angstroms
B)2.30 × 107 angstroms
C)6.89 × 1015 angstroms
D)1.45 × 10-16 angstroms
E)1.31 × 10-9 angstroms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck
80
The frequency of a photon that has an energy of 3.7 × 10-18 J is ________ s-1.
A)5.6 × 1015
B)1.8 × 10-16
C)2.5 × 10-15
D)5.4 × 10-8
E)2.5 × 1015
A)5.6 × 1015
B)1.8 × 10-16
C)2.5 × 10-15
D)5.4 × 10-8
E)2.5 × 1015

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 186 في هذه المجموعة.
فتح الحزمة
k this deck








