Deck 17: Additional Aspects of Aqueous Equilibria
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/116
العب
ملء الشاشة (f)
Deck 17: Additional Aspects of Aqueous Equilibria
1
Of the following solutions, which has the greatest buffering capacity?
A)0.543 M NH3 and 0.555 M NH4Cl
B)0.087 M NH3 and 0.088 M NH4Cl
C)0.234 M NH3 and 0.100 M NH4Cl
D)0.100 M NH3 and 0.455 M NH4Cl
E)They are all buffer solutions and would all have the same capacity.
A)0.543 M NH3 and 0.555 M NH4Cl
B)0.087 M NH3 and 0.088 M NH4Cl
C)0.234 M NH3 and 0.100 M NH4Cl
D)0.100 M NH3 and 0.455 M NH4Cl
E)They are all buffer solutions and would all have the same capacity.
0.543 M NH3 and 0.555 M NH4Cl
2
Which of the following could be added to a solution of potassium fluoride to prepare a buffer?
A)sodium hydroxide
B)potassium acetate
C)hydrochloric acid
D)sodium fluoride
E)ammonia
A)sodium hydroxide
B)potassium acetate
C)hydrochloric acid
D)sodium fluoride
E)ammonia
hydrochloric acid
3
Which one of the following pairs cannot be mixed together to form a buffer solution?
A)HONH2, HONH3Cl
B)NaCl, HCl
C)RbOH, HF
D)KOH, HNO2
E)H2SO3, KHSO3
A)HONH2, HONH3Cl
B)NaCl, HCl
C)RbOH, HF
D)KOH, HNO2
E)H2SO3, KHSO3
NaCl, HCl
4
What change will be caused by addition of a small amount of HCl to a solution containing fluoride ions and hydrogen fluoride?
A)The concentration of hydronium ions will increase significantly.
B)The concentration of fluoride ions will increase as will the concentration of hydronium ions.
C)The concentration of hydrogen fluoride will decrease and the concentration of fluoride ions will increase.
D)The concentration of fluoride ion will decrease and the concentration of hydrogen fluoride will increase.
E)The fluoride ions will precipitate out of solution as its acid salt.
A)The concentration of hydronium ions will increase significantly.
B)The concentration of fluoride ions will increase as will the concentration of hydronium ions.
C)The concentration of hydrogen fluoride will decrease and the concentration of fluoride ions will increase.
D)The concentration of fluoride ion will decrease and the concentration of hydrogen fluoride will increase.
E)The fluoride ions will precipitate out of solution as its acid salt.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
5
A solution containing which one of the following pairs of substances will be a buffer solution?
A)NaI, HI
B)KBr, HBr
C)RbCl, HCl
D)CsF, HF
E)none of the above
A)NaI, HI
B)KBr, HBr
C)RbCl, HCl
D)CsF, HF
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
6
The addition of hydrochloric acid and ________ to water produces a buffer solution.
A)HC6H5O
B)NaOH
C)NH3
D)HNO3
E)NaNO3
A)HC6H5O
B)NaOH
C)NH3
D)HNO3
E)NaNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following could be added to a solution of acetic acid to prepare a buffer?
A)sodium acetate only
B)sodium acetate or sodium hydroxide
C)nitric acid only
D)hydrofluoric acid or nitric acid
E)sodium hydroxide only
A)sodium acetate only
B)sodium acetate or sodium hydroxide
C)nitric acid only
D)hydrofluoric acid or nitric acid
E)sodium hydroxide only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
8
The primary buffer system that controls the pH of the blood is the ________ buffer system.
A)carbon dioxide, carbonate
B)carbonate, bicarbonate
C)carbonic acid, carbon dioxide
D)carbonate, carbonic acid
E)carbonic acid, bicarbonate
A)carbon dioxide, carbonate
B)carbonate, bicarbonate
C)carbonic acid, carbon dioxide
D)carbonate, carbonic acid
E)carbonic acid, bicarbonate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
9
A solution containing which one of the following pairs of substances will be a buffer solution?
A)KI, HI
B)AgBr, HBr
C)CuCl, HCl
D)CsI, HI
E)none of the above
A)KI, HI
B)AgBr, HBr
C)CuCl, HCl
D)CsI, HI
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
10
The addition of sodium hydroxide and ________ to water produces a buffer solution.
A)HCl
B)NaC2H3O2
C)NaF
D)NH3
E)none of the above
A)HCl
B)NaC2H3O2
C)NaF
D)NH3
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
11
The Henderson-Hasselbalch equation is ________.
A)[H+] = Ka +![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2e9_a2ab_cf630f195f89_TB2701_11.jpg)
B)pH = pKa - log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2ea_a2ab_09b92d3b1624_TB2701_11.jpg)
C)pH = pKa + log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2eb_a2ab_6b15c56b101e_TB2701_11.jpg)
D)pH = pKa + log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2ec_a2ab_a5f567fcd90e_TB2701_11.jpg)
E)pH = log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f72_19fd_a2ab_098355407181_TB2701_11.jpg)
A)[H+] = Ka +
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2e9_a2ab_cf630f195f89_TB2701_11.jpg)
B)pH = pKa - log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2ea_a2ab_09b92d3b1624_TB2701_11.jpg)
C)pH = pKa + log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2eb_a2ab_6b15c56b101e_TB2701_11.jpg)
D)pH = pKa + log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f71_f2ec_a2ab_a5f567fcd90e_TB2701_11.jpg)
E)pH = log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f72_19fd_a2ab_098355407181_TB2701_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which one of the following pairs cannot be mixed together to form a buffer solution?
A)NH3, NH4Cl
B)NaC2H3O2, HCl (C2H3O2- = acetate)
C)RbOH, HBr
D)KOH, HF
E)H3PO4, KH2PO4
A)NH3, NH4Cl
B)NaC2H3O2, HCl (C2H3O2- = acetate)
C)RbOH, HBr
D)KOH, HF
E)H3PO4, KH2PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
13
The addition of hydrofluoric acid and ________ to water produces a buffer solution.
A)HCl
B)NaNO3
C)NaCl
D)NaOH
E)NaBr
A)HCl
B)NaNO3
C)NaCl
D)NaOH
E)NaBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
14
The addition of hydrochloric acid and ________ to water produces a buffer solution.
A)HC6H5O
B)NaOH
C)NaCl
D)C2H5NH2
E)none of the above
A)HC6H5O
B)NaOH
C)NaCl
D)C2H5NH2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
15
In a solution, when the concentrations of a weak acid and its conjugate base are equal, ________.
A)the system is not at equilibrium
B)the buffering capacity is significantly decreased
C)the -log of the [H+] and the -log of the Ka are equal
D)All of the above are true.
A)the system is not at equilibrium
B)the buffering capacity is significantly decreased
C)the -log of the [H+] and the -log of the Ka are equal
D)All of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which one of the following pairs cannot be mixed together to form a buffer solution?
A)C5H5N, C5H5NHCl
B)HC2H3O2, NaOH (C2H3O2- = acetate)
C)KOH, HI
D)NH2CH3, HCl
E)NaClO, HNO3
A)C5H5N, C5H5NHCl
B)HC2H3O2, NaOH (C2H3O2- = acetate)
C)KOH, HI
D)NH2CH3, HCl
E)NaClO, HNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following could be added to a solution of acetic acid to prepare a buffer?
A)sodium hydroxide
B)hydrochloric acid
C)nitric acid
D)more acetic acid
E)None of the above can be added to an acetic acid solution to prepare a buffer.
A)sodium hydroxide
B)hydrochloric acid
C)nitric acid
D)more acetic acid
E)None of the above can be added to an acetic acid solution to prepare a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
18
Of the following solutions, which has the greatest buffering capacity?
A)0.521 M HC2H3O2 and 0.217 M NaC2H3O2
B)0.821 M HC2H3O2 and 0.713 M NaC2H3O2
C)0.365M HC2H3O2 and 0.497 M NaC2H3O2
D)0.121 M HC2H3O2 and 0.116 M NaC2H3O2
A)0.521 M HC2H3O2 and 0.217 M NaC2H3O2
B)0.821 M HC2H3O2 and 0.713 M NaC2H3O2
C)0.365M HC2H3O2 and 0.497 M NaC2H3O2
D)0.121 M HC2H3O2 and 0.116 M NaC2H3O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
19
What are the principal organs that regulate the pH of the carbonic acid-bicarbonate buffer system in the blood?
A)kidneys, liver
B)lungs, kidneys
C)spleen, liver
D)lungs, skin
E)brain stem, heart
A)kidneys, liver
B)lungs, kidneys
C)spleen, liver
D)lungs, skin
E)brain stem, heart

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following could be added to a solution of sodium acetate to produce a buffer?
A)acetic acid only
B)acetic acid or hydrochloric acid
C)hydrochloric acid only
D)potassium acetate only
E)sodium chloride or potassium acetate
A)acetic acid only
B)acetic acid or hydrochloric acid
C)hydrochloric acid only
D)potassium acetate only
E)sodium chloride or potassium acetate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
21
The molar solubility of ________ is not affected by the pH of the solution.
A)Na3PO4
B)NaF
C)KNO3
D)AlCl3
E)MnS
A)Na3PO4
B)NaF
C)KNO3
D)AlCl3
E)MnS

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
22
Calculate the pH of a solution prepared by dissolving 0.150 mol of benzoic acid and 0.300 mol of sodium benzoate in water sufficient to yield 1.00 L of solution. The Ka of benzoic acid is 6.30 × 10-5.
A)2.516
B)3.892
C)4.502
D)10.158
E)4.195
A)2.516
B)3.892
C)4.502
D)10.158
E)4.195

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
23
The pH of a solution prepared by dissolving 0.350 mol of solid dimethylamine hydrochloride ((CH3)2NH2Cl)in 1.00 L of 1.10 M dimethylamine ((CH3)2NH)is ________. The Kb for methylamine is 5.40 × 10-4. (Assume the final volume is 1.00 L.)
A)1.66
B)2.77
C)11.23
D)11.14
E)none of the above
A)1.66
B)2.77
C)11.23
D)11.14
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
24
A result of the common-ion effect is ________.
A)that some ions, such as Na+ (aq), frequently appear in solutions but do not participate in solubility equilibria
B)that common ions, such as Na+ (aq), don't affect equilibrium constants
C)that the selective precipitation of a metal ion, such as Ag+, is promoted by the addition of an appropriate counterion (X-)that produces a compound (AgX)with a very low solubility
D) that ions such as K+ and Na+ are common ions, so that their values in equilibrium constant expressions are always 1.00
E)that common ions precipitate all counter-ions
A)that some ions, such as Na+ (aq), frequently appear in solutions but do not participate in solubility equilibria
B)that common ions, such as Na+ (aq), don't affect equilibrium constants
C)that the selective precipitation of a metal ion, such as Ag+, is promoted by the addition of an appropriate counterion (X-)that produces a compound (AgX)with a very low solubility
D) that ions such as K+ and Na+ are common ions, so that their values in equilibrium constant expressions are always 1.00
E)that common ions precipitate all counter-ions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
25
Consider the following table of 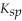 values.
values. 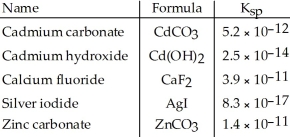
Which compound listed below has the smallest molar solubility in water?
A)ZnCO3
B)Cd(OH)2
C)CdCO3
D)AgI
E)CaF2
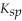 values.
values. 
Which compound listed below has the smallest molar solubility in water?
A)ZnCO3
B)Cd(OH)2
C)CdCO3
D)AgI
E)CaF2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which one of the following will cause hemoglobin to release oxygen?
A)increase in pH
B)decrease in pH
C)decrease in temperature
D)decrease in CO2 concentration
E)increase in O2 concentration
A)increase in pH
B)decrease in pH
C)decrease in temperature
D)decrease in CO2 concentration
E)increase in O2 concentration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
27
The pH of a solution prepared by dissolving 0.350 mol of solid methylamine hydrochloride (CH3NH3Cl)in 1.00 L of 1.10 M methylamine (CH3NH2)is ________. The Kb for methylamine is 4.40 × 10-4. (Assume the final volume is 1.00 L.)
A)1.66
B)2.86
C)10.28
D)11.14
E)10.61
A)1.66
B)2.86
C)10.28
D)11.14
E)10.61

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
28
Why does fluoride treatment render teeth more resistant to decay?
A)Fluoride kills the bacteria in the mouth that make the acids that decay teeth.
B)Fluoride stimulates production of tooth enamel to replace that lost to decay.
C)Fluoride reduces saliva production, keeping teeth drier and thus reducing decay.
D)Fluoride converts hydroxyapatite to fluoroapatite that is less reactive with acids.
E)Fluoride dissolves plaque, reducing its decaying contact with teeth.
A)Fluoride kills the bacteria in the mouth that make the acids that decay teeth.
B)Fluoride stimulates production of tooth enamel to replace that lost to decay.
C)Fluoride reduces saliva production, keeping teeth drier and thus reducing decay.
D)Fluoride converts hydroxyapatite to fluoroapatite that is less reactive with acids.
E)Fluoride dissolves plaque, reducing its decaying contact with teeth.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
29
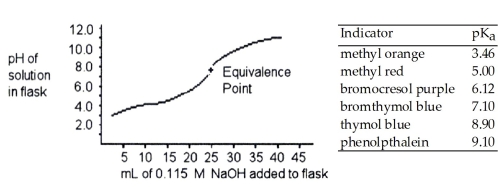
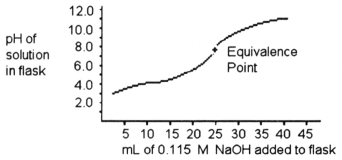
A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. Which of the following indicators would be best for this titration?
A)methyl red
B)bromthymol blue
C)thymol blue
D)phenolpthalein
E)bromocresol purple

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
30
In which one of the following solutions is silver chloride the most soluble?
A)0.181 M HCl
B)0.0176 M NH3
C)0.744 M LiNO3
D)pure water
E)0.181 M NaCl
A)0.181 M HCl
B)0.0176 M NH3
C)0.744 M LiNO3
D)pure water
E)0.181 M NaCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
31
Calculate the pH of a solution prepared by dissolving 0.150 mol of acetic acid and 0.300 mol of sodium acetate in water sufficient to yield 1.00 L of solution. The Ka of acetic acid is 1.76 × 10-5.
A)2.516
B)3.892
C)4.502
D)10.158
E)5.056
A)2.516
B)3.892
C)4.502
D)10.158
E)5.056

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
32
A 25.0 mL sample of a solution of an unknown compound is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The unknown compound is ________.
A)a strong acid
B)a strong base
C)a weak acid
D)a weak base
E)neither an acid nor a base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which below best describe(s)the behavior of an amphoteric hydroxide in water?
A)With conc. aq. NaOH, its suspension dissolves.
B)With conc. aq. HCl, its suspension dissolves.
C)With conc. aq. NaOH, its clear solution forms a precipitate.
D)With conc. aq. HCl, its clear solution forms a precipitate.
E)With both conc. aq. NaOH and conc. aq. HCl, its suspension dissolves.
A)With conc. aq. NaOH, its suspension dissolves.
B)With conc. aq. HCl, its suspension dissolves.
C)With conc. aq. NaOH, its clear solution forms a precipitate.
D)With conc. aq. HCl, its clear solution forms a precipitate.
E)With both conc. aq. NaOH and conc. aq. HCl, its suspension dissolves.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
34
Human blood is ________.
A)neutral
B)very basic
C)slightly acidic
D)very acidic
E)slightly basic
A)neutral
B)very basic
C)slightly acidic
D)very acidic
E)slightly basic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
35
For which salt should the aqueous solubility be most sensitive to pH?
A)Ca(NO3)2
B)CaF2
C)CaCl2
D)CaBr2
E)CaI2
A)Ca(NO3)2
B)CaF2
C)CaCl2
D)CaBr2
E)CaI2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which one of the following is not amphoteric?
A)Al(OH)3
B)Ca(OH)2
C)Cr(OH)3
D)Zn(OH)2
E)Sn(OH)2
A)Al(OH)3
B)Ca(OH)2
C)Cr(OH)3
D)Zn(OH)2
E)Sn(OH)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
37
Consider the following table of 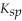 values.
values. 
Which compound listed below has the greatest molar solubility in water?
A)CdCO3
B)Cd(OH)2
C)AgI
D)CaF2
E)ZnCO3
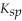 values.
values. 
Which compound listed below has the greatest molar solubility in water?
A)CdCO3
B)Cd(OH)2
C)AgI
D)CaF2
E)ZnCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
38
A 25.0 mL sample of 0.723 M HClO4 is titrated with a 0.27 M KOH solution. The H3O+ concentration after the addition of 80.0 mL of KOH is ________ M.
A)0.4
B)1 × 10-7
C)0.7
D)3 × 10-13
E)4 × 10-2
A)0.4
B)1 × 10-7
C)0.7
D)3 × 10-13
E)4 × 10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
39

A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The concentration of the monoprotic acid is about ________ mol/L.
A)25.0
B)0.0600
C)0.240
D)0.120
E)0.100

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
40
The Ka of benzoic acid is 6.30 × 10-5. The pH of a buffer prepared by combining 50.0 mL of 1.00 M potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
A)1.705
B)0.851
C)3.406
D)4.201
E)2.383
A)1.705
B)0.851
C)3.406
D)4.201
E)2.383

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
41
Calculate the percent ionization of formic acid (HCO2H)in a solution that is 0.152 M in formic acid. The Ka of formic acid is 1.77 × 10-4.
A)2.74 × 10-5
B)0.0180
C)3.44
D)0.581
E)8.44
A)2.74 × 10-5
B)0.0180
C)3.44
D)0.581
E)8.44

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
42
The pH of a solution prepared by mixing 50.0 mL of 0.125 M NaOH and 40.0 mL of 0.125 M HNO3 is ________.
A)13.29
B)7.00
C)8.11
D)11.00
E)12.14
A)13.29
B)7.00
C)8.11
D)11.00
E)12.14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
43
Calculate the maximum concentration (in M)of silver ions (Ag+)in a solution that contains 0.025 M of CO32-. The Ksp of Ag2CO3 is 8.1 × 10-12.
A)1.8 × 10-5
B)1.4 × 10-6
C)2.8 × 10-6
D)3.2 × 10-10
E)8.1 × 10-12
A)1.8 × 10-5
B)1.4 × 10-6
C)2.8 × 10-6
D)3.2 × 10-10
E)8.1 × 10-12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
44
The concentration of iodide ions in a saturated solution of lead (II)iodide is ________ M. The solubility product constant of PbI2 is 1.4 × 10-8.
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-3
D)3.5 × 10-9
E)1.4 × 10-8
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-3
D)3.5 × 10-9
E)1.4 × 10-8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
45
Calculate the pH of a solution that is 0.278 M in sodium formate (NaHCO2)and 0.222 M in formic acid (HCO2H). The Ka of formic acid is 1.77 × 10-4.
A)3.843
B)3.647
C)13.90
D)10.16
E)4.954
A)3.843
B)3.647
C)13.90
D)10.16
E)4.954

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
46
The pH of a solution prepared by mixing 40.0 mL of 0.125 M Mg(OH)2 and 150.0 mL of 0.125 M HCl is ________.
A)6.29
B)4.11
C)1.14
D)5.78
E)1.34
A)6.29
B)4.11
C)1.14
D)5.78
E)1.34

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
47
Calculate the maximum concentration (in M)of magnesium ions (Mg+2)in a solution that contains 0.025 M of CO32-. The Ksp of MgCO3 is 3.5 × 10-8.
A)1.8 × 10-5
B)1.4 × 10-6
C)2.8 × 10-6
D)3.2 × 10-10
E)8.1 × 10-12
A)1.8 × 10-5
B)1.4 × 10-6
C)2.8 × 10-6
D)3.2 × 10-10
E)8.1 × 10-12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
48
Calculate the percent ionization of formic acid (HCO2H)in a solution that is 0.322 M in formic acid and 0.178 M in sodium formate (NaHCO2). The Ka of formic acid is 1.77 × 10-4.
A)35.6
B)0.1011
C)10.8
D)1.03 × 10-3
E)3.488
A)35.6
B)0.1011
C)10.8
D)1.03 × 10-3
E)3.488

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is the solubility (in M)of PbCl2 in a 0.15 M solution of HCl? The Ksp of PbCl2 is 1.6 × 10-5.
A)2.0 × 10-3
B)1.1 × 10-4
C)1.8 × 10-4
D)7.1 × 10-4
E)1.6 × 10-5
A)2.0 × 10-3
B)1.1 × 10-4
C)1.8 × 10-4
D)7.1 × 10-4
E)1.6 × 10-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
50
Calculate the pH of a solution that is 0.322 M in nitrous acid (HNO2)and 0.178 M in potassium nitrite (KNO2). The acid dissociation constant of nitrous acid is 4.50 × 10-4.
A)3.093
B)3.607
C)14.26
D)10.91
E)4.589
A)3.093
B)3.607
C)14.26
D)10.91
E)4.589

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
51
Calculate the percent ionization of nitrous acid in a solution that is 0.241 M in nitrous acid (HNO2)and 0.195 M in potassium nitrite (KNO2). The acid dissociation constant of nitrous acid is 4.50 × 10-4.
A)44.7
B)0.229
C)13.5
D)2.11 × 10-3
E)3.258
A)44.7
B)0.229
C)13.5
D)2.11 × 10-3
E)3.258

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
52
The Ksp for Zn(OH)2 is 5.0 × 10-17. Determine the molar solubility of Zn(OH)2 in a buffer solution with a pH of 11.5.
A)5.0 × 106
B)1.2 × 10-12
C)1.6 × 10-14
D)5.0 × 10-12
E)5.0 × 10-17
A)5.0 × 106
B)1.2 × 10-12
C)1.6 × 10-14
D)5.0 × 10-12
E)5.0 × 10-17

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
53
The pH of a solution prepared by mixing 50.0 mL of 0.125 M KOH and 50.0 mL of 0.125 M HCl is ________.
A)6.29
B)7.00
C)8.11
D)5.78
E)0.00
A)6.29
B)7.00
C)8.11
D)5.78
E)0.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
54
Calculate the percent ionization of nitrous acid in a solution that is 0.260 M in nitrous acid. The acid dissociation constant of nitrous acid is 4.50 × 10-4.
A)1.17 × 10-4
B)0.0450
C)4.16
D)0.314
E)5.78
A)1.17 × 10-4
B)0.0450
C)4.16
D)0.314
E)5.78

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
55
The concentration of iodide ions in a saturated solution of silver iodide is ________ M. The solubility product constant of AgI is 8.3 × 10-17.
A)3.8 × 10-11
B)3.0 × 10-10
C)9.1 × 10-9
D)3.5 × 10-9
E)1.4 × 10-8
A)3.8 × 10-11
B)3.0 × 10-10
C)9.1 × 10-9
D)3.5 × 10-9
E)1.4 × 10-8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
56
The solubility of manganese (II)hydroxide (Mn(OH)2)is 2.2 × 10-5 M. What is the Ksp of Mn(OH)2?
A)1.1 × 10-14
B)4.3 × 10-14
C)2.1 × 10-14
D)4.8 × 10-10
E)2.2 × 10-5
A)1.1 × 10-14
B)4.3 × 10-14
C)2.1 × 10-14
D)4.8 × 10-10
E)2.2 × 10-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
57
The solubility of lead (II)chloride (PbCl2)is 1.6 × 10-2 M. What is the Ksp of PbCl2?
A)5.0 × 10-4
B)4.1 × 10-6
C)3.1 × 10-7
D)1.6 × 10-5
E)1.6 × 10-2
A)5.0 × 10-4
B)4.1 × 10-6
C)3.1 × 10-7
D)1.6 × 10-5
E)1.6 × 10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
58
A 50.0 mL sample of an aqueous H2SO4 solution is titrated with a 0.375 M NaOH solution. The equivalence point is reached with 62.5 mL of the base. The concentration of H2SO4 is ________ M.
A)0.234
B)0.469
C)0.150
D)0.300
E)0.938
A)0.234
B)0.469
C)0.150
D)0.300
E)0.938

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
59
Determine the Ksp for magnesium hydroxide (Mg(OH)2)where the solubility of Mg(OH)2 is 1.4 × 10-4 M.
A)2.7 × 10-12
B)1.1 × 10-11
C)2.0 × 10-8
D)3.9 × 10-8
E)1.4 × 10-4
A)2.7 × 10-12
B)1.1 × 10-11
C)2.0 × 10-8
D)3.9 × 10-8
E)1.4 × 10-4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
60
The concentration of fluoride ions in a saturated solution of barium fluoride is ________ M. The solubility product constant of BaF2 is 1.7 × 10-6.
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-2
D)7.5 × 10-3
E)1.4 × 10-4
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-2
D)7.5 × 10-3
E)1.4 × 10-4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
61
A buffer solution with a pH of 4.63 is prepared with 0.14 M formic acid and ________ M sodium formate. The Ka of formic acid is 1.8 × 10-4.
A)1.1
B)2.1
C)5.4 × 10-6
D)3.0 × 10-8
E)0.54
A)1.1
B)2.1
C)5.4 × 10-6
D)3.0 × 10-8
E)0.54

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
62
A buffer solution with a pH of 4.78 is prepared with ________ M formic acid and 0.90 M sodium formate. The Ka of formic acid is 1.8 × 10-4.
A)0.083
B)0.17
C)3.3 × 103
D)9.8
E)0.041
A)0.083
B)0.17
C)3.3 × 103
D)9.8
E)0.041

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
63
Calculate the pH of a solution prepared by dissolving 0.850 mol of NH3 and 0.300 mol of NH4Cl in water sufficient to yield 1.00 L of solution. The Kb of ammonia is 1.77 × 10-5.
A)5.204
B)4.300
C)9.700
D)8.781
E)8.796
A)5.204
B)4.300
C)9.700
D)8.781
E)8.796

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
64
The pH of a solution that contains 0.800 M acetic acid (Ka = 1.76 × 10-5)and 0.172 M sodium acetate is ________.
A)4.087
B)5.422
C)8.578
D)8.370
E)9.913
A)4.087
B)5.422
C)8.578
D)8.370
E)9.913

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
65
A solution is prepared by dissolving 0.23 mol of hypochlorous acid and 0.27 mol of sodium hypochlorite in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution. The Ka of hypochlorous acid is 1.36 × 10-3.
A)H2O
B)H3O+
C)hypochlorite ion
D)hypochlorous acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.
A)H2O
B)H3O+
C)hypochlorite ion
D)hypochlorous acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
66
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride. The concentration of fluoride ions after the addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is ________ M.
A)0.0735
B)0.0762
C)0.0980
D)0.0709
E)0.00253
A)0.0735
B)0.0762
C)0.0980
D)0.0709
E)0.00253

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
67
A 25.0 mL sample of 0.150 M benzoic acid is titrated with a 0.150 M NaOH solution. What is the pH at the equivalence point? The Ka of benzoic acid is 4.50 × 10-4.
A)11.20
B)9.80
C)4.20
D)7.00
E)8.54
A)11.20
B)9.80
C)4.20
D)7.00
E)8.54

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
68
Calculate the pH of a solution prepared by dissolving 0.250 mol of benzoic acid (C7H5O2H)and 0.150 mol of sodium benzoate (NaC7H5O2)in water sufficient to yield 1.00 L of solution. The Ka of benzoic acid is 6.50 × 10-5.
A)4.409
B)3.965
C)10.035
D)9.591
E)5.190
A)4.409
B)3.965
C)10.035
D)9.591
E)5.190

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the pH of a buffer solution that is 0.172 M in hypochlorous acid (HClO)and 0.131 M in sodium hypochlorite? The Ka of hypochlorous acid is 3.8 × 10-8.
A)14.12
B)6.70
C)9.07
D)7.54
E)7.30
A)14.12
B)6.70
C)9.07
D)7.54
E)7.30

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
70
How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 0.0990 M HBr to the equivalence point?
A)29.5
B)0.332
C)4.57
D)0.208
E)21.2
A)29.5
B)0.332
C)4.57
D)0.208
E)21.2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
71
Calculate the pH of a solution prepared by dissolving 0.270 mol of formic acid (HCO2H)and 0.260 mol of sodium formate (NaCO2H)in water sufficient to yield 1.00 L of solution. The Ka of formic acid is 1.77 × 10-4.
A)2.099
B)10.264
C)3.736
D)2.307
E)3.952
A)2.099
B)10.264
C)3.736
D)2.307
E)3.952

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
72
The Ka of acetic acid is 1.76 × 10-5. The pH of a buffer prepared by combining 15.0 mL of 1.00 M potassium acetate and 50.0 mL of 1.00 M acetic acid is ________.
A)1.705
B)0.851
C)3.406
D)4.232
E)2.383
A)1.705
B)0.851
C)3.406
D)4.232
E)2.383

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
73
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride. The concentration of hydrogen fluoride after addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is ________ M.
A)0.0953
B)0.0900
C)0.130
D)0.122
E)0.00976
A)0.0953
B)0.0900
C)0.130
D)0.122
E)0.00976

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
74
Of the following solutions, which has the greatest buffering capacity?
A)1.15 M HF and 0.624 M NaF
B)0.574 M HF and 0.312 M NaF
C)0.287 M HF and 0.156 M NaF
D)0.189 M HF and 0.103 M NaF
E)They are all buffer solutions and would all have the same capacity.
A)1.15 M HF and 0.624 M NaF
B)0.574 M HF and 0.312 M NaF
C)0.287 M HF and 0.156 M NaF
D)0.189 M HF and 0.103 M NaF
E)They are all buffer solutions and would all have the same capacity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
75
A buffer solution with a pH of 4.31 is prepared with 1.0 M HC2H3O2 and ________ M NaC2H3O2. The Ka of HC2H3O2 is 1.8 × 10-5.
A)0.37
B)0.74
C)4.2 × 10-6
D)8.8 × 10-10
E)0.18
A)0.37
B)0.74
C)4.2 × 10-6
D)8.8 × 10-10
E)0.18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
76
The Kb of ammonia is 1.76 × 10-5. The pH of a buffer prepared by combining 50.0 mL of 1.00 M ammonia and 45.0 mL of 1.00 M ammonium nitrate is ________.
A)4.632
B)9.291
C)4.742
D)9.372
E)none of the above
A)4.632
B)9.291
C)4.742
D)9.372
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
77
A buffer solution with a pH of 4.40 is prepared with 0.78 M Na C2H3O2 and ________ M HC2H3O2. The Ka of HC2H3O2 is 1.8 × 10-5.
A)1.7
B)3.5
C)4.1 × 104
D)0.35
E)0.86
A)1.7
B)3.5
C)4.1 × 104
D)0.35
E)0.86

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
78
The addition of hydrofluoric acid and ________ to water produces a buffer solution.
A)NaF
B)HF
C)NaNO3
D)NaBr
E)KI
A)NaF
B)HF
C)NaNO3
D)NaBr
E)KI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
79
A solution is prepared by dissolving 0.23 mol of benzoic acid and 0.27 mol of sodium benzoate in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly. The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution. The Ka of benzoic acid is 6.3 × 10-5.
A)H2O
B)H3O+
C)benzoate
D)benzoic acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.
A)H2O
B)H3O+
C)benzoate
D)benzoic acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck
80
What is the pH of a buffer solution that is 0.266 M in lactic acid and 0.111 M in sodium lactate? The Ka of lactic acid is 1.4 × 10-4.
A)14.38
B)10.53
C)5.38
D)3.47
E)4.23
A)14.38
B)10.53
C)5.38
D)3.47
E)4.23

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 116 في هذه المجموعة.
فتح الحزمة
k this deck








