Deck 19: Chemical Thermodynamics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/120
العب
ملء الشاشة (f)
Deck 19: Chemical Thermodynamics
1
ΔS is positive for the reaction __________.
A)2NO (g)+ O2 (g)→ 2NO2 (g)
B)2N2 (g)+ 3H2 (g)→ 2 NH3 (g)
C)C3H8 (g)+ 5 O2 (g)→ 3CO2 (g)+ 4 H2O (g)
D)Mg (s)+ Cl2 (g)→ MgCl2 (s)
E)C2H4 (g)+ H2 (g)→ C2H6 (g)
A)2NO (g)+ O2 (g)→ 2NO2 (g)
B)2N2 (g)+ 3H2 (g)→ 2 NH3 (g)
C)C3H8 (g)+ 5 O2 (g)→ 3CO2 (g)+ 4 H2O (g)
D)Mg (s)+ Cl2 (g)→ MgCl2 (s)
E)C2H4 (g)+ H2 (g)→ C2H6 (g)
C3H8 (g)+ 5 O2 (g)→ 3CO2 (g)+ 4 H2O (g)
2
Which one of the following processes produces a decrease of the entropy of the system?
A)dissolving sodium chloride in water
B)sublimation of naphthalene
C)dissolving oxygen in water
D)boiling of alcohol
E)explosion of nitroglycerine
A)dissolving sodium chloride in water
B)sublimation of naphthalene
C)dissolving oxygen in water
D)boiling of alcohol
E)explosion of nitroglycerine
dissolving oxygen in water
3
The thermodynamic quantity that expresses the degree of disorder in a system is __________.
A)enthalpy
B)internal energy
C)bond energy
D)entropy
E)heat flow
A)enthalpy
B)internal energy
C)bond energy
D)entropy
E)heat flow
entropy
4
A reversible process is one that __________.
A)can be reversed with no net change in either system or surroundings
B)happens spontaneously
C)is spontaneous in both directions
D)must be carried out at low temperature
E)must be carried out at high temperature
A)can be reversed with no net change in either system or surroundings
B)happens spontaneously
C)is spontaneous in both directions
D)must be carried out at low temperature
E)must be carried out at high temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
5
The first law of thermodynamics can be given as __________.
A)ΔE = q + w
B)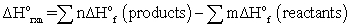
C)for any spontaneous process, the entropy of the universe increases
D)the entropy of a pure crystalline substance at absolute zero is zero
E)ΔS = qrev/T at constant temperature
A)ΔE = q + w
B)
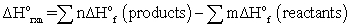
C)for any spontaneous process, the entropy of the universe increases
D)the entropy of a pure crystalline substance at absolute zero is zero
E)ΔS = qrev/T at constant temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
6
The entropy of the universe is __________.
A)constant
B)continually decreasing
C)continually increasing
D)zero
E)the same as the energy, E
A)constant
B)continually decreasing
C)continually increasing
D)zero
E)the same as the energy, E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which one of the following correctly indicates the relationship between the entropy of a system and the number of different arrangements, W, in the system?
A)S = kW
B)S =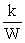
C)S =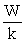
D)S = klnW
E)S = Wk
A)S = kW
B)S =
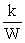
C)S =
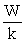
D)S = klnW
E)S = Wk

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which one of the following is always positive when a spontaneous process occurs?
A)ΔSsystem
B)ΔSsurroundings
C)ΔSuniverse
D)ΔHuniverse
E)ΔHsurroundings
A)ΔSsystem
B)ΔSsurroundings
C)ΔSuniverse
D)ΔHuniverse
E)ΔHsurroundings

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following statements is true?
A)Processes that are spontaneous in one direction are spontaneous in the opposite direction.
B)Processes are spontaneous because they occur at an observable rate.
C)Spontaneity can depend on the temperature.
D)All of the statements are true.
A)Processes that are spontaneous in one direction are spontaneous in the opposite direction.
B)Processes are spontaneous because they occur at an observable rate.
C)Spontaneity can depend on the temperature.
D)All of the statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
10
ΔS is positive for the reaction __________.
A)2H2 (g)+ O2 (g)→ 2H2O (g)
B)2NO2 (g)→ N2O4 (g)
C)CO2 (g)→ CO2 (s)
D)BaF2 (s)→ Ba2+ (aq)+ 2F- (aq)
E)2Hg (l)+ O2 (g)→ 2HgO (s)
A)2H2 (g)+ O2 (g)→ 2H2O (g)
B)2NO2 (g)→ N2O4 (g)
C)CO2 (g)→ CO2 (s)
D)BaF2 (s)→ Ba2+ (aq)+ 2F- (aq)
E)2Hg (l)+ O2 (g)→ 2HgO (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
11
Consider a pure crystalline solid that is heated from absolute zero to a temperature above the boiling point of the liquid. Which of the following processes produces the greatest increase in the entropy of the substance?
A)melting the solid
B)heating the liquid
C)heating the gas
D)heating the solid
E)vaporizing the liquid
A)melting the solid
B)heating the liquid
C)heating the gas
D)heating the solid
E)vaporizing the liquid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
12
Of the following, only __________ is not a state function.
A)S
B)H
C)q
D)E
E)T
A)S
B)H
C)q
D)E
E)T

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which one of the following processes produces a decrease in the entropy of the system?
A)boiling water to form steam
B)dissolution of solid KCl in water
C)mixing of two gases into one container
D)freezing water to form ice
E)melting ice to form water
A)boiling water to form steam
B)dissolution of solid KCl in water
C)mixing of two gases into one container
D)freezing water to form ice
E)melting ice to form water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
14
The entropy change accompanying any process is given by the equation:
A)ΔS = k lnWfinal
B)ΔS = k Wfinal - k Winitial
C)ΔS = k ln(Wfinal / Winitial)
D)ΔS = k final - k initial
E)ΔS = Wfinal - Winitial
A)ΔS = k lnWfinal
B)ΔS = k Wfinal - k Winitial
C)ΔS = k ln(Wfinal / Winitial)
D)ΔS = k final - k initial
E)ΔS = Wfinal - Winitial

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
15
For an isothermal process, ΔS = __________.
A)q
B)qrev/T
C)qrev
D)Tqrev
E)q + w
A)q
B)qrev/T
C)qrev
D)Tqrev
E)q + w

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
16
ΔS is positive for the reaction __________.
A)CaO (s)+ CO2 (g)→ CaCO3 (s)
B)N2 (g)+ 3H2 (g)→ 2NH3 (g)
C)2SO3 (g)→ 2SO2 (g)+ O2 (g)
D)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
E)H2O (l)→ H2O (s)
A)CaO (s)+ CO2 (g)→ CaCO3 (s)
B)N2 (g)+ 3H2 (g)→ 2NH3 (g)
C)2SO3 (g)→ 2SO2 (g)+ O2 (g)
D)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
E)H2O (l)→ H2O (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following statements is false?
A)The change in entropy in a system depends on the initial and final states of the system and the path taken from one state to the other.
B)Any irreversible process results in an overall increase in entropy.
C)The total entropy of the universe increases in any spontaneous process.
D)Entropy increases with the number of microstates of the system.
A)The change in entropy in a system depends on the initial and final states of the system and the path taken from one state to the other.
B)Any irreversible process results in an overall increase in entropy.
C)The total entropy of the universe increases in any spontaneous process.
D)Entropy increases with the number of microstates of the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
18
When a system is at equilibrium, __________.
A)the reverse process is spontaneous but the forward process is not
B)the forward and the reverse processes are both spontaneous
C)the forward process is spontaneous but the reverse process is not
D)the process is not spontaneous in either direction
E)both forward and reverse processes have stopped
A)the reverse process is spontaneous but the forward process is not
B)the forward and the reverse processes are both spontaneous
C)the forward process is spontaneous but the reverse process is not
D)the process is not spontaneous in either direction
E)both forward and reverse processes have stopped

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
19
The second law of thermodynamics states that __________.
A)ΔE = q + w
B)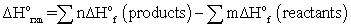
C)for any spontaneous process, the entropy of the universe increases
D)the entropy of a pure crystalline substance is zero at absolute zero
E)ΔS = qrev/T at constant temperature
A)ΔE = q + w
B)
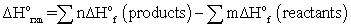
C)for any spontaneous process, the entropy of the universe increases
D)the entropy of a pure crystalline substance is zero at absolute zero
E)ΔS = qrev/T at constant temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
20
A reaction that is spontaneous as written __________.
A)is very rapid
B)will proceed without outside intervention
C)is also spontaneous in the reverse direction
D)has an equilibrium position that lies far to the left
E)is very slow
A)is very rapid
B)will proceed without outside intervention
C)is also spontaneous in the reverse direction
D)has an equilibrium position that lies far to the left
E)is very slow

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
21
For a reaction to be spontaneous under standard conditions at all temperatures, the signs of ΔH° and ΔS° must be __________ and __________, respectively.
A)+, +
B)+, -
C)-, +
D)-, -
E)+, 0
A)+, +
B)+, -
C)-, +
D)-, -
E)+, 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
22
ΔS is negative for the reaction __________.
A)2SO2 (g)+ O2 (g)→ 2SO3 (g)
B)NH4Cl (s)→ NH3 (g)+ HCl (g)
C)PbCl2 (s)→ Pb2+ (aq)+ 2Cl- (aq)
D)2C (s)+ O2 (g)→ 2CO2 (g)
E)H2O (l)→ H2O (g)
A)2SO2 (g)+ O2 (g)→ 2SO3 (g)
B)NH4Cl (s)→ NH3 (g)+ HCl (g)
C)PbCl2 (s)→ Pb2+ (aq)+ 2Cl- (aq)
D)2C (s)+ O2 (g)→ 2CO2 (g)
E)H2O (l)→ H2O (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
23
Of the following, the entropy of __________ is the largest.
A)HCl (l)
B)HCl (s)
C)HCl (g)
D)HBr (g)
E)HI (g)
A)HCl (l)
B)HCl (s)
C)HCl (g)
D)HBr (g)
E)HI (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
24
Given the following table of thermodynamic data,  complete the following sentence. The vaporization of TiCl4 is __________.
complete the following sentence. The vaporization of TiCl4 is __________.
A)spontaneous at all temperatures
B)spontaneous at low temperature and nonspontaneous at high temperature
C)nonspontaneous at low temperature and spontaneous at high temperature
D)nonspontaneous at all temperatures
E)not enough information given to draw a conclusion
 complete the following sentence. The vaporization of TiCl4 is __________.
complete the following sentence. The vaporization of TiCl4 is __________.A)spontaneous at all temperatures
B)spontaneous at low temperature and nonspontaneous at high temperature
C)nonspontaneous at low temperature and spontaneous at high temperature
D)nonspontaneous at all temperatures
E)not enough information given to draw a conclusion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
25
For the reaction C2H6 (g)→ C2H4 (g)+ H2 (g)
ΔH° is + 137 kJ/mol and ΔS° is +120 J/K ∙ mol. This reaction is __________.
A)spontaneous at all temperatures
B)spontaneous only at high temperature
C)spontaneous only at low temperature
D)nonspontaneous at all temperatures
ΔH° is + 137 kJ/mol and ΔS° is +120 J/K ∙ mol. This reaction is __________.
A)spontaneous at all temperatures
B)spontaneous only at high temperature
C)spontaneous only at low temperature
D)nonspontaneous at all temperatures

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
26
Of the following, the entropy of gaseous __________ is the largest at 25°C and 1 atm.
A)CH3OH
B)C2H5OH
C)C3H7OH
D)CH4
E)C4H10
A)CH3OH
B)C2H5OH
C)C3H7OH
D)CH4
E)C4H10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
27
For the reaction 2C4H10 (g)+ 13O2 (g)→ 8CO2 (g)+ 10H2O (g)
ΔH° is -125 kJ/mol and ΔS° is +253 J/K ∙ mol. This reaction is __________.
A)spontaneous at all temperatures
B)spontaneous only at high temperature
C)spontaneous only at low temperature
D)nonspontaneous at all temperatures
E)unable to determine without more information
ΔH° is -125 kJ/mol and ΔS° is +253 J/K ∙ mol. This reaction is __________.
A)spontaneous at all temperatures
B)spontaneous only at high temperature
C)spontaneous only at low temperature
D)nonspontaneous at all temperatures
E)unable to determine without more information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
28
ΔS is negative for the reaction __________.
A)2H2O (g)→ 2H2 (g)+ O2 (g)
B)Mg(NO3)2 (aq)+ 2NaOH(aq)→ Mg(OH)2 (s)+ 2NaNO3 (aq)
C)H2O (l)→ H2O (g)
D)C6H12O6 (s)→ 6C (s)+ 6H2 (g)+ 3O2 (g)
E)NaCl (aq)→ Na+ (aq)+ Cl- (aq)
A)2H2O (g)→ 2H2 (g)+ O2 (g)
B)Mg(NO3)2 (aq)+ 2NaOH(aq)→ Mg(OH)2 (s)+ 2NaNO3 (aq)
C)H2O (l)→ H2O (g)
D)C6H12O6 (s)→ 6C (s)+ 6H2 (g)+ 3O2 (g)
E)NaCl (aq)→ Na+ (aq)+ Cl- (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which reaction produces a decrease in the entropy of the system?
A)4 NH3 (g)+ 5 O2 (g)→ 4 NO (g)+ 6 H2O (g)
B)Na (s)+ 1/2Cl2 (g)→ NaCl (s)
C)2 HgO (s)→ 2 Hg (l)+ O2 (g)
D)U (s)+ 3F2 (g)→ UF6 (s)
E)H2O (s)→ H2O (g)
A)4 NH3 (g)+ 5 O2 (g)→ 4 NO (g)+ 6 H2O (g)
B)Na (s)+ 1/2Cl2 (g)→ NaCl (s)
C)2 HgO (s)→ 2 Hg (l)+ O2 (g)
D)U (s)+ 3F2 (g)→ UF6 (s)
E)H2O (s)→ H2O (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
30
Given the following table of thermodynamic data,  complete the following sentence. The vaporization of PCl3 (l)is __________.
complete the following sentence. The vaporization of PCl3 (l)is __________.
A)nonspontaneous at low temperature and spontaneous at high temperature
B)spontaneous at low temperature and nonspontaneous at high temperature
C)spontaneous at all temperatures
D)nonspontaneous at all temperatures
E)not enough information given to draw a conclusion
 complete the following sentence. The vaporization of PCl3 (l)is __________.
complete the following sentence. The vaporization of PCl3 (l)is __________.A)nonspontaneous at low temperature and spontaneous at high temperature
B)spontaneous at low temperature and nonspontaneous at high temperature
C)spontaneous at all temperatures
D)nonspontaneous at all temperatures
E)not enough information given to draw a conclusion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
31
A reaction that is not spontaneous at low temperature can become spontaneous at high temperature if ΔH is __________ and ΔS is __________.
A)+, +
B)-, -
C)+, -
D)-, +
E)+, 0
A)+, +
B)-, -
C)+, -
D)-, +
E)+, 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
32
ΔS is positive for the reaction __________.
A)2 Ca (s)+ O2 (g)→ 2 CaO (s)
B)2 KClO3 (s)→ 2KCl (s)+ 3 O2 (g)
C)HCl (g)+ NH3 (g)→ NH4Cl (s)
D)Pb+2 (aq)+ 2Cl- (aq)→ PbCl2 (s)
E)CO2 (g)→ CO2 (s)
A)2 Ca (s)+ O2 (g)→ 2 CaO (s)
B)2 KClO3 (s)→ 2KCl (s)+ 3 O2 (g)
C)HCl (g)+ NH3 (g)→ NH4Cl (s)
D)Pb+2 (aq)+ 2Cl- (aq)→ PbCl2 (s)
E)CO2 (g)→ CO2 (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
33
Of the following, the entropy of gaseous __________ is the largest at 25°C and 1 atm.
A)H2
B)C2H6
C)C2H2
D)CH4
E)C2H4
A)H2
B)C2H6
C)C2H2
D)CH4
E)C2H4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
34
The equilibrium position corresponds to which letter on the graph of G vs. f (course of reaction)below? 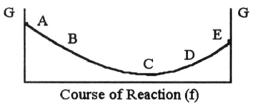
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
35
For an isothermal process, the entropy change of the surroundings is given by the equation:
A)ΔS = qsys T
B)ΔS = -qsys T
C)ΔS = q lnT
D)ΔS = -q lnT
E)ΔS = -qsys/T
A)ΔS = qsys T
B)ΔS = -qsys T
C)ΔS = q lnT
D)ΔS = -q lnT
E)ΔS = -qsys/T

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
36
ΔS is positive for the reaction __________.
A)Pb(NO3)2 (aq)+ 2KI(aq)→ PbI2 (s)+ 2KNO3 (aq)
B)2H2O (g)→ 2H2 (g)+ O2 (g)
C)H2O (g)→ H2O (s)
D)NO (g)+ O2 (g)→ NO2 (g)
E)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
A)Pb(NO3)2 (aq)+ 2KI(aq)→ PbI2 (s)+ 2KNO3 (aq)
B)2H2O (g)→ 2H2 (g)+ O2 (g)
C)H2O (g)→ H2O (s)
D)NO (g)+ O2 (g)→ NO2 (g)
E)Ag+ (aq)+ Cl- (aq)→ AgCl (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which reaction produces an increase in the entropy of the system?
A)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
B)CO2 (s)→ CO2 (g)
C)H2 (g)+ Cl2 (g)→ 2HCl (g)
D)N2 (g)+ 3H2 (g)→ 2NH3 (g)
E)H2O (l)→ H2O (s)
A)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
B)CO2 (s)→ CO2 (g)
C)H2 (g)+ Cl2 (g)→ 2HCl (g)
D)N2 (g)+ 3H2 (g)→ 2NH3 (g)
E)H2O (l)→ H2O (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which reaction produces a decrease in the entropy of the system?
A)CaCO3 (s)→ CaO (s)+ CO2 (g)
B)2C (s)+ O2 (g)→ 2CO (g)
C)CO2 (s)→ CO2 (g)
D)2H2 (g)+ O2 (g)→ 2H2O (l)
E)H2O (l)→ H2O (g)
A)CaCO3 (s)→ CaO (s)+ CO2 (g)
B)2C (s)+ O2 (g)→ 2CO (g)
C)CO2 (s)→ CO2 (g)
D)2H2 (g)+ O2 (g)→ 2H2O (l)
E)H2O (l)→ H2O (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
39
Consider the reaction: Ag+ (aq)+ Cl- (aq)→ AgCl (s)
Given the following table of thermodynamic data,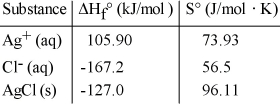 determine the temperature (in °C)above which the reaction is nonspontaneous under standard conditions.
determine the temperature (in °C)above which the reaction is nonspontaneous under standard conditions.
A)1230
B)150
C)432
D)133
E)1640
Given the following table of thermodynamic data,
 determine the temperature (in °C)above which the reaction is nonspontaneous under standard conditions.
determine the temperature (in °C)above which the reaction is nonspontaneous under standard conditions.A)1230
B)150
C)432
D)133
E)1640

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
40
Of the following, the entropy of __________ is the largest.
A)H2O (s)
B)H2O (l)
C)H2O (g)
D)H2S (g)
E)H2S (l)
A)H2O (s)
B)H2O (l)
C)H2O (g)
D)H2S (g)
E)H2S (l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
41
The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water: C2H4 (g)+ 3O2 (g)→ 2CO2 (g)+ 2H2O (l)
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)-267.4
B)-140.9
C)-347.6
D)+347.6
E)+140.9
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)-267.4
B)-140.9
C)-347.6
D)+347.6
E)+140.9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
42
The value of ΔS° for the catalytic hydrogenation of ethene to ethane, C2H4 (g)+ H2(g)→ C2H6 (g)
Is __________ J/K∙ mol.
A)-101.9
B)-120.5
C)-232.5
D)+112.0
E)+101.9
Is __________ J/K∙ mol.
A)-101.9
B)-120.5
C)-232.5
D)+112.0
E)+101.9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
43
The value of ΔS° for the reaction 2C (s, diamond)+ O2 (g)→ 2CO (g)
Is __________ J/K∙ mol.
A)-185.9
B)+185.9
C)-9.5
D)+9.5
E)-195.7
Is __________ J/K∙ mol.
A)-185.9
B)+185.9
C)-9.5
D)+9.5
E)-195.7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
44
The combustion of hydrogen in the presence of excess oxygen yields water: 2H2(g)+ O2(g)→ 2H2O (l)
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)+405.5
B)-405.5
C)-326.3
D)-265.7
E)+265.7
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)+405.5
B)-405.5
C)-326.3
D)-265.7
E)+265.7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
45
The value of ΔS° for the oxidation of carbon to carbon dioxide, C (s, graphite)+ O2 (g)→ CO2(g)
Is __________ J/K∙ mol. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
A)+424.3
B)+205.0
C)-205.0
D)-2.9
E)+2.9
Is __________ J/K∙ mol. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
A)+424.3
B)+205.0
C)-205.0
D)-2.9
E)+2.9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
46
If ΔG° for a reaction is greater than zero, then __________.
A)K = 0
B)K = 1
C)K > 1
D)K < 1
E)More information is needed.
A)K = 0
B)K = 1
C)K > 1
D)K < 1
E)More information is needed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
47
The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, S (s, rhombic)+ O2(g)→ SO2(g)
Is __________ J/K∙ mol.
A)+485.4
B)+248.5
C)-11.6
D)-248.5
E)+11.6
Is __________ J/K∙ mol.
A)+485.4
B)+248.5
C)-11.6
D)-248.5
E)+11.6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
48
The value of ΔS° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2 (g)→ S (s, rhombic)+ O2 (g)
Is __________ J/K∙ mol.
A)+485.4
B)+248.5
C)-11.6
D)-248.5
E)+11.6
Is __________ J/K∙ mol.
A)+485.4
B)+248.5
C)-11.6
D)-248.5
E)+11.6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
49
The value of ΔS° for the formation of POCl3 from its constituent elements, P2 (g)+ O2 (g)+ 3Cl2 (g)→ 2POCl3 (g)
Is __________ J/K∙ mol.
A)-442.0
B)+771.0
C)-321.0
D)-771.0
E)+321.0
Is __________ J/K∙ mol.
A)-442.0
B)+771.0
C)-321.0
D)-771.0
E)+321.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
50
Consider the reaction: NH3 (g)+ HCl (g)→ NH4Cl (s)
Given the following table of thermodynamic data, determine the temperature (in °C)above which the reaction is nonspontaneous.
determine the temperature (in °C)above which the reaction is nonspontaneous.
A)This reaction is spontaneous at all temperatures.
B)618.1
C)432.8
D)345.0
E)1235
Given the following table of thermodynamic data,
 determine the temperature (in °C)above which the reaction is nonspontaneous.
determine the temperature (in °C)above which the reaction is nonspontaneous.A)This reaction is spontaneous at all temperatures.
B)618.1
C)432.8
D)345.0
E)1235

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
51
The value of ΔS° for the oxidation of carbon to carbon monoxide, 2C (s, graphite)+ O2 (g)→ 2CO (g)
Is __________ J/K∙ mol. Carbon monoxide is produced in the combustion of carbon with limited oxygen.
A)-12.8
B)+408.6
C)-408.6
D)+179.4
E)+395.8
Is __________ J/K∙ mol. Carbon monoxide is produced in the combustion of carbon with limited oxygen.
A)-12.8
B)+408.6
C)-408.6
D)+179.4
E)+395.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
52
The value of ΔS° for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2SO3 (g)→ 2S (s, rhombic)+ 3O2 (g)
Is __________ J/K∙ mol.
A)+19.3
B)-19.3
C)+493.1
D)+166.4
E)-493.1
Is __________ J/K∙ mol.
A)+19.3
B)-19.3
C)+493.1
D)+166.4
E)-493.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
53
Consider the reaction: FeO (s)+ Fe (s)+ O2 (g)→ Fe2O3 (s)
Given the following table of thermodynamic data,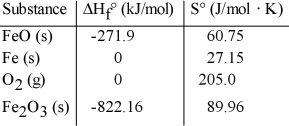 determine the temperature (in °C)above which the reaction is nonspontaneous.
determine the temperature (in °C)above which the reaction is nonspontaneous.
A)This reaction is spontaneous at all temperatures.
B)618.1
C)756.3
D)2438
E)1235
Given the following table of thermodynamic data,
 determine the temperature (in °C)above which the reaction is nonspontaneous.
determine the temperature (in °C)above which the reaction is nonspontaneous.A)This reaction is spontaneous at all temperatures.
B)618.1
C)756.3
D)2438
E)1235

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
54
The combustion of acetylene in the presence of excess oxygen yields carbon dioxide and water: 2C2H2 (g)+ 5O2 (g)→ 4CO2 (g)+ 2H2O (l)
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)+689.3
B)+122.3
C)+432.4
D)-122.3
E)-432.4
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)+689.3
B)+122.3
C)+432.4
D)-122.3
E)-432.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
55
The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic)+ 3O2(g)→ 2SO3 (g)
Is __________ J/K∙ mol.
A)+19.3
B)-19.3
C)+493.1
D)-166.4
E)-493.1
Is __________ J/K∙ mol.
A)+19.3
B)-19.3
C)+493.1
D)-166.4
E)-493.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which one of the following statements is true about the equilibrium constant for a reaction if ΔG° for the reaction is negative?
A)K = 0
B)K = 1
C)K > 1
D)K < 1
E)More information is needed.
A)K = 0
B)K = 1
C)K > 1
D)K < 1
E)More information is needed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
57
The combustion of ethane in the presence of excess oxygen yields carbon dioxide and water: 2C2H6 (g)+ 7O2 (g)→ 4CO2 (g)+ 6H2O (l)
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)+718.0
B)-620.1
C)-718.0
D)-151.0
E)+151.0
The value of ΔS° for this reaction is __________ J/K∙ mol.
A)+718.0
B)-620.1
C)-718.0
D)-151.0
E)+151.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
58
With thermodynamics, one cannot determine __________.
A)the speed of a reaction
B)the direction of a spontaneous reaction
C)the extent of a reaction
D)the value of the equilibrium constant
E)the temperature at which a reaction will be spontaneous
A)the speed of a reaction
B)the direction of a spontaneous reaction
C)the extent of a reaction
D)the value of the equilibrium constant
E)the temperature at which a reaction will be spontaneous

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
59
The value of ΔS° for the catalytic hydrogenation of acetylene to ethene, C2H2 (g)+ H2 (g)→ C2H4 (g)
Is __________ J/K∙ mol.
A)+18.6
B)+550.8
C)+112.0
D)-112.0
E)-18.6
Is __________ J/K∙ mol.
A)+18.6
B)+550.8
C)+112.0
D)-112.0
E)-18.6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
60
The value of ΔS° for the catalytic hydrogenation of acetylene to ethane, C2H2 (g)+ 2H2 (g)→ C2H6 (g)
Is __________ J/K∙ mol.
A)-76.0
B)+440.9
C)-232.5
D)+232.5
E)+28.7
Is __________ J/K∙ mol.
A)-76.0
B)+440.9
C)-232.5
D)+232.5
E)+28.7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
61
The value of ΔS° for the decomposition of POCl3 into its constituent elements, 2POCl3 (g)→ P2 (g)+ O2 (g)+ 3Cl2 (g)
Is __________ J/K∙ mol.
A)+771.0
B)+442.0
C)-321.0
D)-771.0
E)+321.0
Is __________ J/K∙ mol.
A)+771.0
B)+442.0
C)-321.0
D)-771.0
E)+321.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
62
The value of ΔS° for the formation of phosphorous trichloride from its constituent elements, P2 (g)+ 3Cl2 (g)→ 2PCl3 (g)
Is __________ J/K∙ mol.
A)-311.7
B)+311.7
C)-263.6
D)+129.4
E)-129.4
Is __________ J/K∙ mol.
A)-311.7
B)+311.7
C)-263.6
D)+129.4
E)-129.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
63
The value of ΔH° for the formation of calcium chloride from its constituent elements, Ca (s)+ Cl2 (g)→ CaCl2 (s)
Is __________ kJ/mol.
A)+0.00
B)-397.9
C)+397.9
D)-795.8
E)+795.8
Is __________ kJ/mol.
A)+0.00
B)-397.9
C)+397.9
D)-795.8
E)+795.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
64
The value of ΔH° for the formation of phosphorous trichloride from its constituent elements, P2 (g)+ 3Cl2 (g)→ 2PCl3 (g)
Is __________ kJ/mol
A)-288.1
B)+432.4
C)-720.5
D)+720.5
E)-432.4
Is __________ kJ/mol
A)-288.1
B)+432.4
C)-720.5
D)+720.5
E)-432.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
65
The value of ΔH° for the formation of POCl3 from its constituent elements, P2 (g)+ O2 (g)+ 3Cl2 (g)→ 2POCl3 (g)
Is __________ kJ/mol.
A)-1228.7
B)-397.7
C)-686.5
D)+1228.7
E)+686.5
Is __________ kJ/mol.
A)-1228.7
B)-397.7
C)-686.5
D)+1228.7
E)+686.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
66
The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, S (s, rhombic)+ O2(g)→ SO2 (g)
Is __________ kJ/mol.
A)+395.2
B)+269.9
C)-269.9
D)+300.4
E)-300.4
Is __________ kJ/mol.
A)+395.2
B)+269.9
C)-269.9
D)+300.4
E)-300.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
67
The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g)→ P2 (g)+ 3Cl2 (g)
Is __________ kJ/mol.
A)+576.2
B)-288.1
C)+720.5
D)+288.1
E)-720.5
Is __________ kJ/mol.
A)+576.2
B)-288.1
C)+720.5
D)+288.1
E)-720.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
68
The value of ΔG° at 25°C for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2SO3 (g)→ 2S (s, rhombic)+ 3O2 (g)
Is __________ kJ/mol.
A)+740.8
B)-370.4
C)+370.4
D)-740.8
E)+185.2
Is __________ kJ/mol.
A)+740.8
B)-370.4
C)+370.4
D)-740.8
E)+185.2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
69
The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic)+ 3O2( g)→ 2SO3 (g)
Is __________ kJ/mol.
A)+790.4
B)-790.4
C)+395.2
D)-395.2
E)+105.1
Is __________ kJ/mol.
A)+790.4
B)-790.4
C)+395.2
D)-395.2
E)+105.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
70
The value of ΔH° for the decomposition of calcium chloride into its constituent elements, CaCl2 (s)→ Ca (s)+ Cl2 (g)
Is __________ kJ/mol.
A)-0.00
B)-397.9
C)+397.9
D)-795.8
E)+795.8
Is __________ kJ/mol.
A)-0.00
B)-397.9
C)+397.9
D)-795.8
E)+795.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
71
The value of ΔH° for the decomposition of POCl3 into its constituent elements, 2POCl3 (g)→ P2 (g)+ O2 (g)+ 3Cl2 (g)
Is __________ kJ/mol.
A)-1228.7
B)+1228.7
C)-940.1
D)+940.1
E)+0.00
Is __________ kJ/mol.
A)-1228.7
B)+1228.7
C)-940.1
D)+940.1
E)+0.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
72
The value of ΔS° for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g)→ P2 (g)+ 3Cl2( g)
Is __________ J/K∙ mol.
A)-311.7
B)+311.7
C)+263.6
D)+129.4
E)-129.4
Is __________ J/K∙ mol.
A)-311.7
B)+311.7
C)+263.6
D)+129.4
E)-129.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
73
The value of ΔH° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2 (g)→ S (s,rhombic)+ O2 (g)
Is __________ kJ/mol.
A)+0.0
B)+135.0
C)-135.90
D)-269.9
E)+269.9
Is __________ kJ/mol.
A)+0.0
B)+135.0
C)-135.90
D)-269.9
E)+269.9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
74
The value of ΔG° at 25°C for the formation of POCl3 from its constituent elements, P2 (g)+ O2 (g)+ 3Cl2 (g)→ 2POCl3 (g)
Is __________ kJ/mol.
A)-1108.7
B)+1108.7
C)-606.2
D)+606.2
E)-1,005
Is __________ kJ/mol.
A)-1108.7
B)+1108.7
C)-606.2
D)+606.2
E)-1,005

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
75
The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic)+ 3O2 (g)→ 2SO3 (g)
Is __________ kJ/mol.
A)+740.8
B)-370.4
C)+370.4
D)-740.8
E)+185.2
Is __________ kJ/mol.
A)+740.8
B)-370.4
C)+370.4
D)-740.8
E)+185.2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
76
The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, S (s, rhombic)+ O2 (g)→ SO2 (g)
Is __________ kJ/mol.
A)+269.9
B)-269.9
C)+0.00
D)-11.6
E)+11.6
Is __________ kJ/mol.
A)+269.9
B)-269.9
C)+0.00
D)-11.6
E)+11.6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
77
The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements, 2SO3 (g)→ 2S (s, rhombic)+ 3O2 (g)
Is __________ kJ/mol.
A)+790.4
B)-790.4
C)+395.2
D)-395.2
E)+105.1
Is __________ kJ/mol.
A)+790.4
B)-790.4
C)+395.2
D)-395.2
E)+105.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
78
The value of ΔG° at 25°C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2 (g)→ S (s, rhombic)+ O2 (g)
Is __________ kJ/mol.
A)+395.2
B)+269.9
C)-269.9
D)+300.4
E)-300.4
Is __________ kJ/mol.
A)+395.2
B)+269.9
C)-269.9
D)+300.4
E)-300.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
79
The value of ΔS° for the decomposition of calcium chloride into its constituent elements, CaCl2 (s)→ Ca (s)+ Cl2 (g)
Is __________ J/K∙ mol.
A)-104.6
B)+104.6
C)+369.0
D)-159.8
E)+159.8
Is __________ J/K∙ mol.
A)-104.6
B)+104.6
C)+369.0
D)-159.8
E)+159.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
80
The value of ΔS° for the formation of calcium chloride from its constituent elements, Ca (s)+ Cl2 (g)→ CaCl2 (s)
Is __________ J/K∙ mol.
A)-104.6
B)+104.6
C)+369.0
D)-159.8
E)+159.8
Is __________ J/K∙ mol.
A)-104.6
B)+104.6
C)+369.0
D)-159.8
E)+159.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck








