Deck 17: Carboxylic Acids and Their Derivatives
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/89
العب
ملء الشاشة (f)
Deck 17: Carboxylic Acids and Their Derivatives
1
Which acid would be expected to have the highest boiling point?
A)acetic,CH3CO2H
B)benzoic,C6H5CO2H
C)formic,HCO2H
D)oxalic, (CO2H)2
E)stearic,CH3(CH2)16 CO2H
A)acetic,CH3CO2H
B)benzoic,C6H5CO2H
C)formic,HCO2H
D)oxalic, (CO2H)2
E)stearic,CH3(CH2)16 CO2H
stearic,CH3(CH2)16 CO2H
2
All of the statements about carboxylic acids are true except
A)they undergo substitution reactions involving the -OH group.
B)at low molecular weights they are liquids with sharp stinging odors.
C)they form hydrogen bonds,causing their boiling points to be higher than expected on the basis of molecular weight.
D)they react with bases to form salts which are often more soluble than the original acid.
E)when they behave as acids,the -OH group is lost leaving the CO- ion.
A)they undergo substitution reactions involving the -OH group.
B)at low molecular weights they are liquids with sharp stinging odors.
C)they form hydrogen bonds,causing their boiling points to be higher than expected on the basis of molecular weight.
D)they react with bases to form salts which are often more soluble than the original acid.
E)when they behave as acids,the -OH group is lost leaving the CO- ion.
when they behave as acids,the -OH group is lost leaving the CO- ion.
3
Which molecule is an ester?
A)
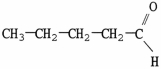
B)
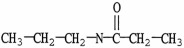
C)
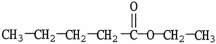
D)
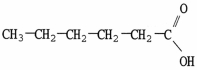
E)C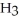
C
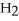
C
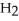
N
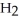
A)
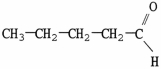
B)
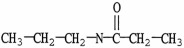
C)
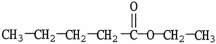
D)
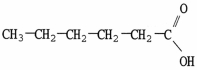
E)C
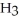
C
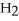
C
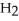
N
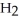
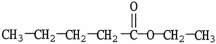
4
Which compound is a carboxylic acid?
A)CH3COO-K+
B)CH3COOH
C)(CH3)2CHOOCH3
D)(CH3CO)2O
E)(CH3)2O
A)CH3COO-K+
B)CH3COOH
C)(CH3)2CHOOCH3
D)(CH3CO)2O
E)(CH3)2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following is(are)neither an acid or a base?
A)amines
B)carboxylic acids
C)esters
D)amides
E)both C and D
A)amines
B)carboxylic acids
C)esters
D)amides
E)both C and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which molecule is a carboxylic acid?
A)
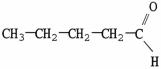
B)
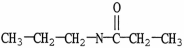
C)
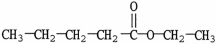
D)
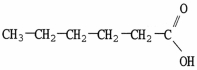
E)C
C
C
N
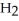
A)
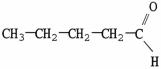
B)
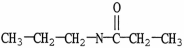
C)
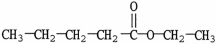
D)
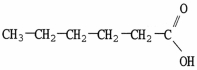
E)C
C
C
N
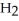

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
7
All of the statements concerning citric acid are true except
A)it contains three carboxylic acid groups.
B)it is very soluble in water.
C)its salts are used in many consumer products.
D)it is produced only by plants.
E)it is a weak acid.
A)it contains three carboxylic acid groups.
B)it is very soluble in water.
C)its salts are used in many consumer products.
D)it is produced only by plants.
E)it is a weak acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
8
The similarities in properties of carboxylic acids,esters,and amides can best be explained by
A)their polarity and structural similarities.
B)their similarities in molar mass.
C)hydrogen bonding.
D)the ease with which they form ions.
E)none of these
A)their polarity and structural similarities.
B)their similarities in molar mass.
C)hydrogen bonding.
D)the ease with which they form ions.
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which acid would be expected to have the lowest boiling point?
A)acetic,CH3CO2H
B)benzoic,C6H5CO2H
C)formic,HCO2H
D)oxalic, (CO2H)2
E)stearic,CH3(CH2)16CO2H
A)acetic,CH3CO2H
B)benzoic,C6H5CO2H
C)formic,HCO2H
D)oxalic, (CO2H)2
E)stearic,CH3(CH2)16CO2H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
10
The pleasant,characteristic odor of fruit flavorings is often associated with the presence of
A)esters.
B)carboxylic acids.
C)carboxylate salts.
D)ketones.
A)esters.
B)carboxylic acids.
C)carboxylate salts.
D)ketones.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon atom?
A)aldehyde
B)amide
C)carboxylic acid
D)ester
E)ketone
A)aldehyde
B)amide
C)carboxylic acid
D)ester
E)ketone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which functional group contains a carbonyl group and an ether linkage bonded to the same carbon atom?
A)aldehyde
B)amide
C)carboxylic acid
D)ester
E)ketone
A)aldehyde
B)amide
C)carboxylic acid
D)ester
E)ketone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following functional groups cannot hydrogen bond with itself?
A)carboxylic acids
B)esters
C)primary amines
D)alcohols
E)primary amides
A)carboxylic acids
B)esters
C)primary amines
D)alcohols
E)primary amides

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
14
Carboxylic acids generally taste
A)sour.
B)sweet.
C)bitter.
D)salty.
E)spicy.
A)sour.
B)sweet.
C)bitter.
D)salty.
E)spicy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
15
An acyl group is a group in which a(n)
A)amine and a carbonyl are bonded to the same carbon atom.
B)hydroxyl and an alkene are bonded to the same carbon atom.
C)alpha carbon is bonded to an alkyl group.
D)alkyl group is bonded to a carbonyl carbon atom.
E)acidic group is bonded to an aromatic group.
A)amine and a carbonyl are bonded to the same carbon atom.
B)hydroxyl and an alkene are bonded to the same carbon atom.
C)alpha carbon is bonded to an alkyl group.
D)alkyl group is bonded to a carbonyl carbon atom.
E)acidic group is bonded to an aromatic group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
16
The functional groups in the aspirin molecule shown are 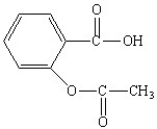
A)aromatic;carboxylic acid.
B)aromatic;ester.
C)carboxylic acid;ester.
D)aromatic;carboxylic acid;ester.
E)amide;aromatic;carboxylic acid.

A)aromatic;carboxylic acid.
B)aromatic;ester.
C)carboxylic acid;ester.
D)aromatic;carboxylic acid;ester.
E)amide;aromatic;carboxylic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following bonds is not present in a carboxylic acid functional group?
A)C=C
B)C=O
C)C-O
D)O-H
E)none of the above
A)C=C
B)C=O
C)C-O
D)O-H
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
18
The most common reactions of carboxylic acids or their derivatives involve
A)addition across the double bond between carbon and oxygen.
B)replacement of the oxygen atom in the carbonyl group.
C)replacement of the group bonded to the carbonyl atom.
D)oxidation of the R group.
E)reduction of the R group.
A)addition across the double bond between carbon and oxygen.
B)replacement of the oxygen atom in the carbonyl group.
C)replacement of the group bonded to the carbonyl atom.
D)oxidation of the R group.
E)reduction of the R group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following has the highest boiling point?
A)ethane,CH3CH3
B)dimethyl ketone,CH3COCH3
C)ethyl alcohol,CH3CH2OH
D)acetic acid,CH3COOH
E)formaldehyde,HCHO
A)ethane,CH3CH3
B)dimethyl ketone,CH3COCH3
C)ethyl alcohol,CH3CH2OH
D)acetic acid,CH3COOH
E)formaldehyde,HCHO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which molecule is an amide?
A)
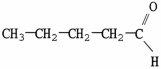
B)
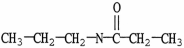
C)

D)
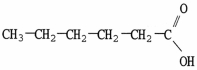
E)C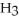
C
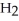
C
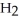
N
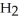
A)
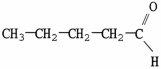
B)
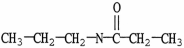
C)

D)
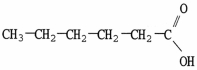
E)C
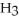
C
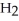
C
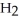
N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the IUPAC name of the compound shown? 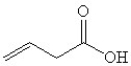
A)1-butanoic acid
B)4-butanoic acid
C)1-butenoic acid
D)3-butenoic acid
E)none of the above

A)1-butanoic acid
B)4-butanoic acid
C)1-butenoic acid
D)3-butenoic acid
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the IUPAC name of the molecule shown? 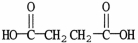
A)diethanoic acid
B)ethanedioc
C)dibutanoic acid
D)butanedioic acid
E)pentanedioic acid
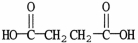
A)diethanoic acid
B)ethanedioc
C)dibutanoic acid
D)butanedioic acid
E)pentanedioic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the correct structure for the compound isopropylbenzoate?
A)
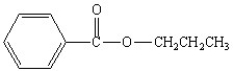
B)
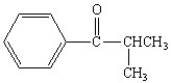
C)
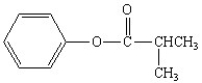
D)
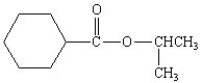
E)
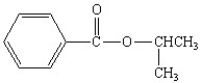
A)
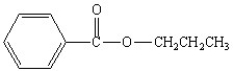
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
24
The solubility of compounds containing the carboxylic acid group can be increased by reaction with
A)sulfuric acid.
B)nitric acid.
C)sodium hydroxide.
D)water.
E)benzoic acid.
A)sulfuric acid.
B)nitric acid.
C)sodium hydroxide.
D)water.
E)benzoic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which molecule is oxalic acid?
A)
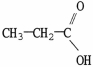
B)
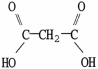
C)
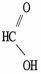
D)
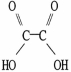
E)
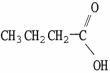
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which molecule shown is β-hydroxy butyric acid?
A)

B)

C)

D)

E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which equation correctly represents the dissociation of a carboxylic acid in water?
A)CH3COOH + H2O
CH3CHCOOH2+ + OH-
B)CH3COOH
CH3COO- + H+
C)CH3COOH + H2O
CH3COO- + H3O+
D)CH3COOH + H3O+
CH3COOH2+ + H2O
E)CH3COOH + 2H2O
CH3COO2- + 2H3O+
A)CH3COOH + H2O

CH3CHCOOH2+ + OH-
B)CH3COOH

CH3COO- + H+
C)CH3COOH + H2O

CH3COO- + H3O+
D)CH3COOH + H3O+

CH3COOH2+ + H2O
E)CH3COOH + 2H2O

CH3COO2- + 2H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the IUPAC name of the compound shown? 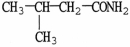
A)2-methyl propanamide
B)N-methyl propanamide
C)2-methyl butanamide
D)3-methyl butanamide
E)N-methyl butanamide
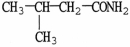
A)2-methyl propanamide
B)N-methyl propanamide
C)2-methyl butanamide
D)3-methyl butanamide
E)N-methyl butanamide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the compounds shown can form hydrogen bonds with other identical molecules? Explain each case. 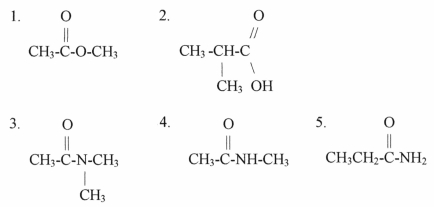


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the IUPAC name of the molecule shown? 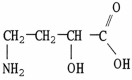
A)γ-amino-α-hydroxybutyric acid
B)4-amino-2-hydroxybutanoic acid
C)α-amino-γ-hydroxybutyric acid
D)1-amino-3-hydroxybutanoic acid
E)none of these

A)γ-amino-α-hydroxybutyric acid
B)4-amino-2-hydroxybutanoic acid
C)α-amino-γ-hydroxybutyric acid
D)1-amino-3-hydroxybutanoic acid
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
31
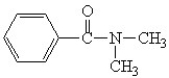
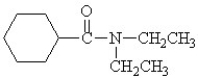
Which is the correct structure for N,N-diethyl benzamide?
A)
B)
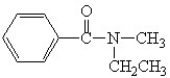
C)
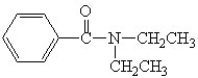
D)
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which molecule is acetic acid?
A)
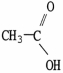
B)
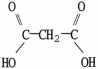
C)
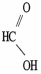
D)
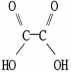
E)
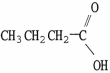
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the IUPAC name of the compound shown? 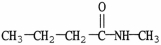
A)1-methylpropanamide
B)N-methylpropanamide
C)N-butylethanamide
D)N-methylbutanamide
E)N-butylformamide
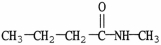
A)1-methylpropanamide
B)N-methylpropanamide
C)N-butylethanamide
D)N-methylbutanamide
E)N-butylformamide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the common name of the molecule shown? 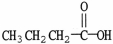
A)acetic acid
B)butyric acid
C)formic acid
D)lactic acid
E)oxalic acid
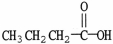
A)acetic acid
B)butyric acid
C)formic acid
D)lactic acid
E)oxalic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the IUPAC name of the compound shown? 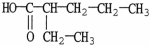
A)3-hexanoic acid
B)2-ethyl pentanoate
C)2-ethylpentanoic acid
D)3-heptanoic acid
E)4-heptanoic acid
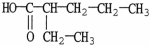
A)3-hexanoic acid
B)2-ethyl pentanoate
C)2-ethylpentanoic acid
D)3-heptanoic acid
E)4-heptanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the IUPAC name of the compound shown?
CH3CH2COOCH3
A)2-butanoic acid
B)3-butanoic acid
C)methyl ethanoate
D)propyl methanoate
E)methyl propanoate
CH3CH2COOCH3
A)2-butanoic acid
B)3-butanoic acid
C)methyl ethanoate
D)propyl methanoate
E)methyl propanoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
37
An alpha hydroxy carboxylic acid has an additional -OH group attached to the molecule at which location?
A)the carbonyl carbon atom
B)the #2 carbon atom
C)the carbon atom farthest from the carboxyl group
D)the carbon atom that contains the amine group
E)none of the above
A)the carbonyl carbon atom
B)the #2 carbon atom
C)the carbon atom farthest from the carboxyl group
D)the carbon atom that contains the amine group
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the IUPAC name of the molecule shown? 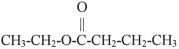
A)ethyl butanoate
B)butyl ethanoate
C)acetyl butyrate
D)butyl acetate
E)2-hexanoic ester
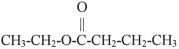
A)ethyl butanoate
B)butyl ethanoate
C)acetyl butyrate
D)butyl acetate
E)2-hexanoic ester

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which is the correct structure for 3-methyl butanamide?
A)
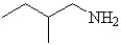
B)
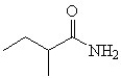
C)
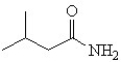
D)
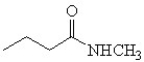
E)
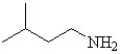
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the IUPAC name for the following compound? 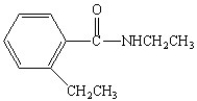
A)N,2-diethyl benzamide
B)diethyl benzamide
C)N,N-diethyl benzamide
D)ethyl phenyl amine
E)diethyl phenyl amine

A)N,2-diethyl benzamide
B)diethyl benzamide
C)N,N-diethyl benzamide
D)ethyl phenyl amine
E)diethyl phenyl amine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
41
Reaction of a carboxylic acid with a base like sodium hydroxide,NaOH gives a(n)
A)carboxylate salt.
B)alkoxide salt.
C)ester.
D)alcohol.
E)none of the above
A)carboxylate salt.
B)alkoxide salt.
C)ester.
D)alcohol.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
42
What two compounds should be used to make N-methylbutanamide?
A)ammonia and butanoic acid
B)methyl amine and butanoic acid
C)ammonia and 1-butanol
D)methyl amine and 1-butanol
E)ammonia and methylbutanoate
A)ammonia and butanoic acid
B)methyl amine and butanoic acid
C)ammonia and 1-butanol
D)methyl amine and 1-butanol
E)ammonia and methylbutanoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
43
The ion formed from a carboxylic acid is called the
A)carboxylate anion.
B)carboxylate cation.
C)ester anion.
D)ester cation.
E)amide cation.
A)carboxylate anion.
B)carboxylate cation.
C)ester anion.
D)ester cation.
E)amide cation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
44
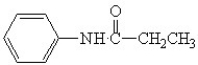
In order to synthesize N-phenyl acetamide what should you combine aniline with?
A)butanoic acid
B)acetic acid
C)methyl amine
D)hydrocholoric acid
E)benzoic acid
A)butanoic acid
B)acetic acid
C)methyl amine
D)hydrocholoric acid
E)benzoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the major organic product of the reaction shown? 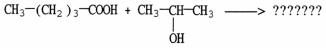
A)
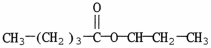
B)
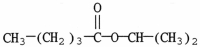
C)
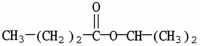
D)
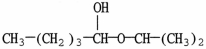
E)
CH3CH2CH2CH2OCH2OCH(CH3)2
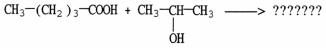
A)
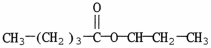
B)
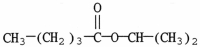
C)
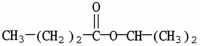
D)

E)
CH3CH2CH2CH2OCH2OCH(CH3)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which carboxylic acid is used to prepare the ester shown? 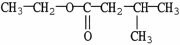
A)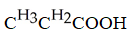
B)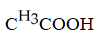
C)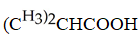
D)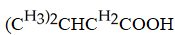
E)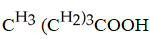
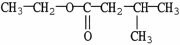
A)
B)
C)
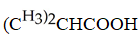
D)
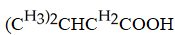
E)
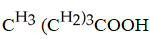

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
47
The common chemical name of aspirin is
A)acetamide.
B)acetylsalicylic acid.
C)phenylalanyl aspartic acid.
D)acetaminophen.
E)lidocaine.
A)acetamide.
B)acetylsalicylic acid.
C)phenylalanyl aspartic acid.
D)acetaminophen.
E)lidocaine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following acids is the strongest?
A)acetic acid,pKa = 4.74
B)hexanoic acid,pKa = 4.89
C)formic acid,pKa = 3.74
D)oxalic acid,pKa1 = 1.27
E)carbonic acid,pKa1 = 6.35
A)acetic acid,pKa = 4.74
B)hexanoic acid,pKa = 4.89
C)formic acid,pKa = 3.74
D)oxalic acid,pKa1 = 1.27
E)carbonic acid,pKa1 = 6.35

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
49
The products of basic hydrolysis of an ester are
A)another ester + water.
B)alcohol + acid.
C)alcohol + water.
D)acid + water.
E)carboxylate salt + alcohol.
A)another ester + water.
B)alcohol + acid.
C)alcohol + water.
D)acid + water.
E)carboxylate salt + alcohol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
50
Predict the products and write the complete reaction between propanoic acid and potassium hydroxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
51
When an alcohol reacts with a carboxylic acid the major product is a(n)
A)amide.
B)amine.
C)ester.
D)salt.
E)soap.
A)amide.
B)amine.
C)ester.
D)salt.
E)soap.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following conditions would favor the carboxylate ion form of a carboxylic acid?
A)low pH
B)high pH
C)both A and B
D)neither A nor B
A)low pH
B)high pH
C)both A and B
D)neither A nor B

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
53
What are the major organic products of the reaction shown? 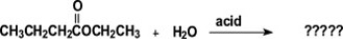
A)CH3CH2CH2COOH + HOCH2CH3
B)CH3CH2CH2COO- + H2OCH2CH3
C)CH3COOH + HOCH2CH2CH2CH3
D)CH3CH2CH2CH2OH + CH3CH2OH
E)CH3CH2CH2COOH + CH3COOH
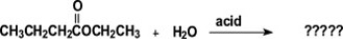
A)CH3CH2CH2COOH + HOCH2CH3
B)CH3CH2CH2COO- + H2OCH2CH3
C)CH3COOH + HOCH2CH2CH2CH3
D)CH3CH2CH2CH2OH + CH3CH2OH
E)CH3CH2CH2COOH + CH3COOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
54
The products of acid hydrolysis of an ester are
A)another ester + water.
B)alcohol + acid.
C)alcohol + water.
D)acid + water.
E)salt + water.
A)another ester + water.
B)alcohol + acid.
C)alcohol + water.
D)acid + water.
E)salt + water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of these compounds is the most soluble in water?
A)CH3CH2CH2CH2CH2COOH
B)CH3CH2CH2CH2CH2COO-Na+
C)CH3CH2CH2CH2CH2OH
D)CH3CH2CH2CH2CH2CH3
E)CH3CH2OCH2CH2CH3
A)CH3CH2CH2CH2CH2COOH
B)CH3CH2CH2CH2CH2COO-Na+
C)CH3CH2CH2CH2CH2OH
D)CH3CH2CH2CH2CH2CH3
E)CH3CH2OCH2CH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
56
Reaction of an ester with a strong base is called
A)reverse esterification.
B)esterification.
C)saponification.
D)oxidation.
E)condensation.
A)reverse esterification.
B)esterification.
C)saponification.
D)oxidation.
E)condensation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which formula correctly illustrates the form which acetic acid would take in a basic solution?
A)
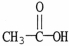
B)
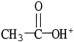
C)
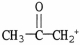
D)
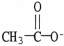
E)
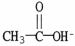
A)
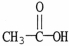
B)
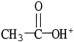
C)
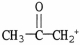
D)
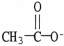
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
58
When an amine reacts with a carboxylic acid at high temperature the major product is a(n)
A)ester.
B)amide.
C)thiol.
D)ether.
E)alcohol.
A)ester.
B)amide.
C)thiol.
D)ether.
E)alcohol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
59
In the production of an ester,the carboxylic acid loses which atom or group of atoms?
A)oxygen from the C=O
B)H from the OH group
C)the OH group
D)the entire COOH group
E)oxygen from the OH
A)oxygen from the C=O
B)H from the OH group
C)the OH group
D)the entire COOH group
E)oxygen from the OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
60
Reaction of butanoic acid with ethanol produces
A)butyl ethanoate.
B)ethyl butanoate.
C)butyl ethanamide.
D)ethyl butanamide.
E)butyl ethyl ester.
A)butyl ethanoate.
B)ethyl butanoate.
C)butyl ethanamide.
D)ethyl butanamide.
E)butyl ethyl ester.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
61
Match the following.
amide group
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol
amide group
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
62
The reactants needed to produce simple polyamides (nylons)are
A)diacids and dialcohols.
B)diacids and diamines.
C)diamines and dialcohols.
D)alkenes and catalysts.
E)diacids and phosphates.
A)diacids and dialcohols.
B)diacids and diamines.
C)diamines and dialcohols.
D)alkenes and catalysts.
E)diacids and phosphates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
63
Match the following.
ester
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol
ester
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which of the following might be obtained as one of the products in this reaction? 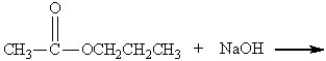
A)
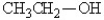
B)
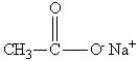
C)
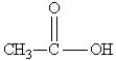
D)
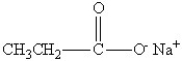
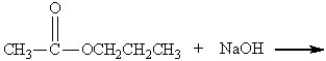
A)
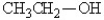
B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
65
The amide produced from pentanoic acid and ammonia is
A)N-pentanamide.
B)N-methylpentanamide.
C)pentanoicamide.
D)pentanamide.
A)N-pentanamide.
B)N-methylpentanamide.
C)pentanoicamide.
D)pentanamide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
66
Match the following.
methanoic acid
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol
methanoic acid
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
67
Nylons and proteins are both referred to as polyamides because
A)each reactant molecule contains an amide group.
B)they are produced by reaction between an amide and an ester.
C)they are formed when an acid reacts with ammonia.
D)they are produced by basic hydrolysis of an amine.
E)they are formed when an acid functional group reacts with an amine functional group.
A)each reactant molecule contains an amide group.
B)they are produced by reaction between an amide and an ester.
C)they are formed when an acid reacts with ammonia.
D)they are produced by basic hydrolysis of an amine.
E)they are formed when an acid functional group reacts with an amine functional group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
68
This compound is 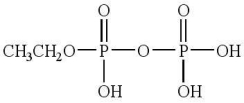
A)an anhydride only.
B)an ester only.
C)both an ester and an anhydride.
D)neither an ester nor an anhydride.
E)phosphoric acid.

A)an anhydride only.
B)an ester only.
C)both an ester and an anhydride.
D)neither an ester nor an anhydride.
E)phosphoric acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
69
The reactants needed to produce simple polyesters are
A)diacids and dialcohols.
B)diacids and diamines.
C)diamines and dialcohols.
D)alkenes and catalysts.
E)diacids and phosphates.
A)diacids and dialcohols.
B)diacids and diamines.
C)diamines and dialcohols.
D)alkenes and catalysts.
E)diacids and phosphates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
70
Phosphate esters are prepared from
A)phosphoric acid + alcohol.
B)phosphoric acid + ester.
C)phosphoric acid + carboxylic acid.
D)phosphate + ester.
E)phosphate + alcohol.
A)phosphoric acid + alcohol.
B)phosphoric acid + ester.
C)phosphoric acid + carboxylic acid.
D)phosphate + ester.
E)phosphate + alcohol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
71
Match the following.
ethanoic acid
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol
ethanoic acid
A)a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B)a functional group consisting of an amine group bonded to a carbonyl carbon
C)the carbon atom bonded directly to the carbonyl carbon atom
D)a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E)the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F)a functional group formed when two acid molecules give up one water molecule
G)the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H)a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I)The common name of this compound is acetic acid.
J)The common name of this compound is formic acid.
K)a polymer produced by reacting diamines with diacids or diacyl chlorides
L)the transfer of a phosphoryl group from one molecule to another
M)the type of ester produced when phosphoric acid reacts with three molecules of alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
72
The two monomers used to make Dacron polyester fabric are
A)ethylene glycol and mylar.
B)terephthalic acid and mylar.
C)propylene glycol and mylar.
D)ethylene glycol and terephthalic acid.
E)none of the above
A)ethylene glycol and mylar.
B)terephthalic acid and mylar.
C)propylene glycol and mylar.
D)ethylene glycol and terephthalic acid.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
73
When an amide is hydrolyzed under acidic conditions,the products are an
A)amine and a carboxylic acid.
B)amine and a carboxylate ion.
C)ammonium ion and a carboxylate ion.
D)ammonium ion and a carboxylic acid.
E)There is no reaction.
A)amine and a carboxylic acid.
B)amine and a carboxylate ion.
C)ammonium ion and a carboxylate ion.
D)ammonium ion and a carboxylic acid.
E)There is no reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
74
What are the products of this reaction? 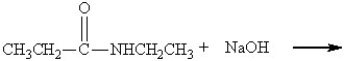
A)
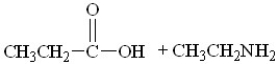
B)
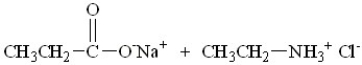
C)
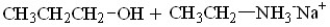
D)
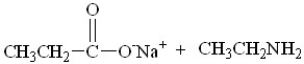
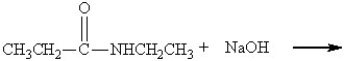
A)
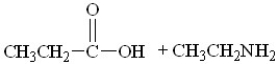
B)
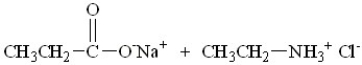
C)
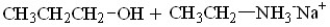
D)
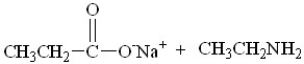

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
75
One requirement for the reactants in the formation of polyester is that each molecule contain
A)at least one carbon-carbon double bond.
B)one aromatic ring.
C)at least two functional groups that can form ester linkages.
D)an amine group somewhere on the carbon skeleton.
E)none of these
A)at least one carbon-carbon double bond.
B)one aromatic ring.
C)at least two functional groups that can form ester linkages.
D)an amine group somewhere on the carbon skeleton.
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
76
The potassium or sodium salt of a long chain carboxylic acid is called a
A)soap.
B)triglyceride.
C)ester.
D)emollient.
E)none of the above
A)soap.
B)triglyceride.
C)ester.
D)emollient.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
77
Hydrolysis of a carboxylic acid ester using base is called
A)saponification.
B)decarboxylation.
C)detoxification.
D)alcoholysis.
E)extraction.
A)saponification.
B)decarboxylation.
C)detoxification.
D)alcoholysis.
E)extraction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
78
When an amide is hydrolyzed under basic conditions,the products are an
A)amine and a carboxylic acid.
B)amine and a carboxylate ion.
C)ammonium ion and a carboxylate ion.
D)ammonium ion and a carboxylic acid.
E)There is no reaction.
A)amine and a carboxylic acid.
B)amine and a carboxylate ion.
C)ammonium ion and a carboxylate ion.
D)ammonium ion and a carboxylic acid.
E)There is no reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
79
Hydrolysis of the ester ethyl acetate produces
A)ethanol and acetic acid.
B)butanol.
C)butanal and ethanol.
D)ethanal and acetic acid.
E)butanoic acid.
A)ethanol and acetic acid.
B)butanol.
C)butanal and ethanol.
D)ethanal and acetic acid.
E)butanoic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
80
When an alcohol reacts with phosphoric acid,the product is referred to as a
A)phosphate salt.
B)phosphate ester.
C)phosphate anion.
D)pyrophosphate.
E)none of the above
A)phosphate salt.
B)phosphate ester.
C)phosphate anion.
D)pyrophosphate.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck








