Deck 8: Gases, liquids, and Solids
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/90
العب
ملء الشاشة (f)
Deck 8: Gases, liquids, and Solids
1
Which transformation is condensation?
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid
gas → liquid
2
Which combination of characteristics is most likely to be associated with molecules having strong dipole-dipole interactions?
I.polar bond
II.asymmetric shape
III.relatively low boiling point
IV.large surface area
A)I and III
B)II and IV
C)I and II
D)III and IV
E)I,II,III,and IV
I.polar bond
II.asymmetric shape
III.relatively low boiling point
IV.large surface area
A)I and III
B)II and IV
C)I and II
D)III and IV
E)I,II,III,and IV
I and II
3
Which process is endothermic?
A)Water condenses on the outside of a cold soda can.
B)The melted wax hardens after a candle is extinguished.
C)Water vapor forms ice crystals in the upper atmosphere.
D)Gasoline spilled on the ground evaporates very quickly.
E)none of the above
A)Water condenses on the outside of a cold soda can.
B)The melted wax hardens after a candle is extinguished.
C)Water vapor forms ice crystals in the upper atmosphere.
D)Gasoline spilled on the ground evaporates very quickly.
E)none of the above
Gasoline spilled on the ground evaporates very quickly.
4
Which process is exothermic?
A)gas → liquid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)none of the above
A)gas → liquid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which is the best description of hydrogen bonding?
A)the unique chemical bonds between hydrogen and any other atom in the same molecule
B)the association between hydrogen of one molecule and a region of another molecule which has become negative due to temporary shifts in electron density
C)the polarity associated with a bond between hydrogen and a small electronegative atom to which it is bonded
D)the association between a hydrogen atom which is somewhat positive because it is bonded to a small electronegative atom and an atom of O,N or F on another molecule
E)the temporary attraction between hydrogen atoms on different molecules resulting from shifts in electron density
A)the unique chemical bonds between hydrogen and any other atom in the same molecule
B)the association between hydrogen of one molecule and a region of another molecule which has become negative due to temporary shifts in electron density
C)the polarity associated with a bond between hydrogen and a small electronegative atom to which it is bonded
D)the association between a hydrogen atom which is somewhat positive because it is bonded to a small electronegative atom and an atom of O,N or F on another molecule
E)the temporary attraction between hydrogen atoms on different molecules resulting from shifts in electron density

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which molecule will undergo only London dispersion forces when interacting with other molecules of the same kind?
A)HF
B)CH2Cl2
C)C4H10
D)NaC2H3O2
E)C2H5OH
A)HF
B)CH2Cl2
C)C4H10
D)NaC2H3O2
E)C2H5OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which pair of molecules has the strongest dipole-dipole interactions?
A)NH3 and CH4
B)NH3 and NH3
C)CH4 and CH4
D)CO2 and CO2
E)CO2 and CH4
A)NH3 and CH4
B)NH3 and NH3
C)CH4 and CH4
D)CO2 and CO2
E)CO2 and CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which description best fits a solid?
A)definite shape and volume;strong intermolecular attractions
B)definite volume;shape of container;moderate intermolecular attractions
C)definite volume;shape of container;no intermolecular attractions
D)volume and shape of container;no intermolecular attractions
E)volume and shape of container;strong intermolecular attractions
A)definite shape and volume;strong intermolecular attractions
B)definite volume;shape of container;moderate intermolecular attractions
C)definite volume;shape of container;no intermolecular attractions
D)volume and shape of container;no intermolecular attractions
E)volume and shape of container;strong intermolecular attractions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which transformation is melting?
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
10
In comparing gases with liquids,gases have ________ compressibility and ________ density.
A)greater;smaller
B)greater;greater
C)smaller;smaller
D)smaller;greater
E)none of the above
A)greater;smaller
B)greater;greater
C)smaller;smaller
D)smaller;greater
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which transformation is evaporation?
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → solid
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → solid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which description best fits a liquid?
A)definite shape and volume;strong intermolecular attractions
B)definite volume;shape of container;moderate intermolecular attractions
C)definite volume;shape of container;no intermolecular attractions
D)volume and shape of container;no intermolecular attractions
E)volume and shape of container;strong intermolecular attractions
A)definite shape and volume;strong intermolecular attractions
B)definite volume;shape of container;moderate intermolecular attractions
C)definite volume;shape of container;no intermolecular attractions
D)volume and shape of container;no intermolecular attractions
E)volume and shape of container;strong intermolecular attractions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
13
What are the strongest intermolecular forces between pentane (C5H12)molecules?
A)dipole-dipole
B)ion-dipole
C)london dispersion forces
D)hydrogen bonding
E)ionic bonds
A)dipole-dipole
B)ion-dipole
C)london dispersion forces
D)hydrogen bonding
E)ionic bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which transformation is freezing?
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which transformation is sublimation?
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid
A)liquid → solid
B)liquid → gas
C)solid → liquid
D)solid → gas
E)gas → liquid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which intermolecular force is characteristic of compounds with low molar mass,which are liquids at room temperature and have relatively high boiling points?
A)dipole-dipole forces
B)London forces
C)hydrogen bonds
D)ionic bonds
E)covalent bonds
A)dipole-dipole forces
B)London forces
C)hydrogen bonds
D)ionic bonds
E)covalent bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following cannot have hydrogen bonds?
A)H2O
B)HCl
C)HF
D)CH3NH2
E)NH3
A)H2O
B)HCl
C)HF
D)CH3NH2
E)NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
18
The process of sublimation is ________ and involves a(an)________ in entropy.
A)exothermic;increase
B)exothermic;decrease
C)endothermic;increase
D)endothermic;decrease
E)endothermic;no change
A)exothermic;increase
B)exothermic;decrease
C)endothermic;increase
D)endothermic;decrease
E)endothermic;no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which description best fits a gas?
A)definite shape and volume;strong intermolecular attractions
B)definite volume;shape of container;moderate intermolecular attractions
C)definite volume;shape of container;weak intermolecular attractions
D)volume and shape of container;no intermolecular attractions
E)volume and shape of container;strong intermolecular attractions
A)definite shape and volume;strong intermolecular attractions
B)definite volume;shape of container;moderate intermolecular attractions
C)definite volume;shape of container;weak intermolecular attractions
D)volume and shape of container;no intermolecular attractions
E)volume and shape of container;strong intermolecular attractions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
20
In comparing liquids and gases,liquids have ________ compressibility and ________ density.
A)smaller;smaller
B)smaller;greater
C)greater;smaller
D)greater;greater
E)none of the above
A)smaller;smaller
B)smaller;greater
C)greater;smaller
D)greater;greater
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
21
What would be the new pressure if a 400 mL gas sample at 380 mm Hg is expanded to 800 mL with no change in temperature?
A)190 mm Hg
B)380 mm Hg
C)570 mm Hg
D)760 mm Hg
E)950 mm Hg
A)190 mm Hg
B)380 mm Hg
C)570 mm Hg
D)760 mm Hg
E)950 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the assumptions of the kinetic-molecular theory best explains the observation that a balloon collapses when exposed to liquid nitrogen (which is much colder than a cold winter day!!)?
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which represents the largest pressure?
A)one atmosphere
B)five pounds per square inch
C)one millimeter of mercury
D)five mm Hg
E)one hundred pascals
A)one atmosphere
B)five pounds per square inch
C)one millimeter of mercury
D)five mm Hg
E)one hundred pascals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which is not a correct statement of Charles's Law?
A)For a gas sample at constant pressure,temperature and volume are inversely proportional.
B)volume α temperature
C)V/T = a constant
D)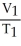
=
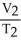
E)none of the above
A)For a gas sample at constant pressure,temperature and volume are inversely proportional.
B)volume α temperature
C)V/T = a constant
D)
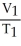
=
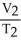
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
25
Rank the following in increasing order of increasing boiling point:
N2 CH3CH2OH CO2 HCl
A)CH3CH2OH < HCl < N2 < CO2
B)HCl < CH3CH2OH < N2 < CO2
C)N2 < CO2 < HCl < CH3CH2OH
D)CH3CH2OH < HCl < CO2 < N2
E)N2 < CO2 < CH3CH2OH < HCl
N2 CH3CH2OH CO2 HCl
A)CH3CH2OH < HCl < N2 < CO2
B)HCl < CH3CH2OH < N2 < CO2
C)N2 < CO2 < HCl < CH3CH2OH
D)CH3CH2OH < HCl < CO2 < N2
E)N2 < CO2 < CH3CH2OH < HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
26
The barometric pressure given in the local weather report is reported as inHg.What is the pressure in mmHg if the weatherman says the current pressure today is 29.75 in Hg?
A)75.57 mmHg
B)755.7 mmHg
C)117.1 mmHg
D)945.0 mmHg
E)760.0 mmHg
A)75.57 mmHg
B)755.7 mmHg
C)117.1 mmHg
D)945.0 mmHg
E)760.0 mmHg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which measurement represents the largest value of pressure?
A)0.750 atm
B)785 mm Hg
C)755 torr
D)22.0 psi
E)100 kPa
A)0.750 atm
B)785 mm Hg
C)755 torr
D)22.0 psi
E)100 kPa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
28
Using the kinetic molecular theory,as the temperature of a gas rises the pressure will also increase because
A)the gas molecules collide more frequently with the wall of container.
B)the gas molecules collide less frequently with the wall of a container.
C)the gas molecules collide more energetically with the wall of the container.
D)both the gas molecules collide more frequently and more energetically with the wall of the container.
A)the gas molecules collide more frequently with the wall of container.
B)the gas molecules collide less frequently with the wall of a container.
C)the gas molecules collide more energetically with the wall of the container.
D)both the gas molecules collide more frequently and more energetically with the wall of the container.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which is not a correct statement of Boyle's Law?
A)For a gas sample at constant temperature,pressure and volume are inversely proportional.
B)pressure α 1/volume
C)pressure × volume = a constant
D)P1V1 = P2V2
E)none of the above
A)For a gas sample at constant temperature,pressure and volume are inversely proportional.
B)pressure α 1/volume
C)pressure × volume = a constant
D)P1V1 = P2V2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the assumptions of the kinetic-molecular theory best explains the observation that a gas can be compressed?
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
31
Arrange the following in order of increasing intermolecular forces:
N2 CH3CH2OH CO2 HCl
A)N2 < CO2 < HCl < CH3CH2OH
B)CH3CH2OH < HCl < N2 < CO2
C)CH3CH2OH < HCl < CO2 < N2
D)HCl < CH3CH2OH < N2 < CO2
E)N2 < CO2 < CH3CH2OH < HCl
N2 CH3CH2OH CO2 HCl
A)N2 < CO2 < HCl < CH3CH2OH
B)CH3CH2OH < HCl < N2 < CO2
C)CH3CH2OH < HCl < CO2 < N2
D)HCl < CH3CH2OH < N2 < CO2
E)N2 < CO2 < CH3CH2OH < HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
32
When the volume of a gas is plotted against the Celsius temperature while holding pressure and the number of moles constant,the x-intercept will occur at what temperature?
A)0°C
B)-273°C
C)the boiling point of the gas
D)the melting point of the gas
E)100°C
A)0°C
B)-273°C
C)the boiling point of the gas
D)the melting point of the gas
E)100°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
33
Volume and pressure are ________ proportional.
A)directly
B)inversely
C)all of the above
D)none of the above
A)directly
B)inversely
C)all of the above
D)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which assumption of Kinetic Molecular Theory is not followed when a real gas shows non-ideal behavior?
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the assumptions of the kinetic-molecular theory best explains Dalton's law of partial pressures?
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.
A)Gas molecules move at random with no attractive forces between them.
B)The velocity of gas molecules is proportional to their Kelvin temperature.
C)The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules.
D)In collisions with the walls of the container or with other molecules,energy is conserved.
E)Collisions with the walls of the container or with other molecules are elastic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which measurement represents the smallest value of pressure?
A)0.750 atm
B)785 mm Hg
C)755 torr
D)22.0 psi
E)100 kPa
A)0.750 atm
B)785 mm Hg
C)755 torr
D)22.0 psi
E)100 kPa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the new volume of a balloon originally at 755 torr and 5.00 L is placed in a container in which the pressure is increased to 1.25 atm?
A)6.29 L
B)3.97 L
C)0.159 L
D)302 L
E)39.7 L
A)6.29 L
B)3.97 L
C)0.159 L
D)302 L
E)39.7 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
38
Most gases show variations from ideality at ________ pressure and ________ temperature.
A)high;low
B)low;high
C)high;high
D)low;low
E)moderate;moderate
A)high;low
B)low;high
C)high;high
D)low;low
E)moderate;moderate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
39
Sketch a graph illustrating Boyle's Law.Use a gas sample with a volume of 2.0 L at 0.75 atm and 25°C,and show at least two other points.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
40
The number of mm Hg in one atmosphere is
A)14.7.
B)760.
C)1.00.
D)29.9.
E)101.325.
A)14.7.
B)760.
C)1.00.
D)29.9.
E)101.325.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
41
A sample of helium has a volume of 480 mL at 47.0°C and 740 mm Hg.The temperature is lowered to 22.0°C and the pressure to 625 mm Hg.What is the new volume?
A)266 mL
B)373 mL
C)524 mL
D)616 mL
E)1214 mL
A)266 mL
B)373 mL
C)524 mL
D)616 mL
E)1214 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
42
A balloon originally had a volume of 4.39 L at 44°C and a pressure of 729 torr.To what temperature must the balloon be cooled to reduce its volume to 3.78 L if the pressure is constant?
A)38°C
B)0.0°C
C) 72.9 °C
D)273°C
E)237°C
A)38°C
B)0.0°C
C) 72.9 °C
D)273°C
E)237°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
43
How will the volume of a fixed sample of gas change if the pressure is doubled and its Celsius temperature is halved?
A)The change cannot be determined without more specific information.
B)It will double.
C)It will decrease by a factor of 4.
D)It will decrease by a factor of 2.
E)It will remain constant.
A)The change cannot be determined without more specific information.
B)It will double.
C)It will decrease by a factor of 4.
D)It will decrease by a factor of 2.
E)It will remain constant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following is the definition of standard temperature and pressure?
A)273°C and 760 torr
B)298 K and 1 atm
C)273 K and 760 mm Hg
D)0 K and 1 atm
A)273°C and 760 torr
B)298 K and 1 atm
C)273 K and 760 mm Hg
D)0 K and 1 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
45
For the following reaction,if 11.2 L of nitrogen are reacted to form NH3 at STP,how many liters of hydrogen will be required to completely consume all of the nitrogen?
N2 + 3H2 → 2NH3
A)16.8 L
B)11.2 L
C)7.4 L
D)33.6 L
E)6.1 L
N2 + 3H2 → 2NH3
A)16.8 L
B)11.2 L
C)7.4 L
D)33.6 L
E)6.1 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
46
How will the volume of a fixed sample of gas change if its pressure is doubled and the Kelvin temperature is doubled?
A)The change cannot be determined without more specific information.
B)It will double.
C)It will increase by a factor of 4.
D)It will decrease by a factor of 2.
E)It will not change.
A)The change cannot be determined without more specific information.
B)It will double.
C)It will increase by a factor of 4.
D)It will decrease by a factor of 2.
E)It will not change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
47
A sealed container with gas at 2.00 atm is heated from 20.0 K to 40.0 K.The new pressure is
A)0.500 atm.
B)1.00 atm.
C)4.00 atm.
D)2.14 atm.
E)1.87 atm.
A)0.500 atm.
B)1.00 atm.
C)4.00 atm.
D)2.14 atm.
E)1.87 atm.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
48
If the temperature of a 1.75 liter sample of gas is changed from 30.0°C to 20.0°C at constant pressure,what will be the new volume?
A)1.17 L
B)1.57 L
C)1.69 L
D)1.81 L
E)2.63 L
A)1.17 L
B)1.57 L
C)1.69 L
D)1.81 L
E)2.63 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
49
A 250.mL sample of gas at 1.00 atm and 20.0°C has the temperature increased to 40.0°C and the volume increased to 500.mL.What is the new pressure?
A)0.374 atm
B)0.468 atm
C)0.534 atm
D)1.87 atm
E)2.14 atm
A)0.374 atm
B)0.468 atm
C)0.534 atm
D)1.87 atm
E)2.14 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
50
According to Avogadro's Law,the volume of a gas will ________ as the ________ is increased while the ________ are held constant.
A)increase;number of moles;pressure and temperature
B)decrease;number of moles;pressure and temperature
C)increase;temperature;pressure and number of moles
D)decrease;pressure;temperature and number of moles
E)increase;pressure;temperature and number of moles
A)increase;number of moles;pressure and temperature
B)decrease;number of moles;pressure and temperature
C)increase;temperature;pressure and number of moles
D)decrease;pressure;temperature and number of moles
E)increase;pressure;temperature and number of moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
51
What will be the new volume when 128 mL of gas at 20.0°C is heated to 40.0°C while pressure remains unchanged?
A)64.0 mL
B)120.mL
C)128 mL
D)137 mL
E)256 mL
A)64.0 mL
B)120.mL
C)128 mL
D)137 mL
E)256 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which is a correct statement of Gay-Lussac's Law describing the behavior of a fixed amount of gas?
A)As pressure increases,volume increases at constant temperature.
B)As pressure increases,volume decreases at constant temperature.
C)As temperature increases,pressure decreases at constant volume.
D)As temperature increases,pressure increases at constant volume.
E)As temperature increases,volume increases at constant pressure.
A)As pressure increases,volume increases at constant temperature.
B)As pressure increases,volume decreases at constant temperature.
C)As temperature increases,pressure decreases at constant volume.
D)As temperature increases,pressure increases at constant volume.
E)As temperature increases,volume increases at constant pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
53
Chlorine gas is widely used to purify municipal water supplies and to treat swimming pool waters.Suppose that the volume of a particular sample of Cl2 is 718 mL at 675 mmHg and 48°C,at what temperature (in °C)will the volume be 2.00 L if the pressure is 159 kPa?
A)1308°C
B)273°C
C)237°C
D)1581°C
E)-36.6°C
A)1308°C
B)273°C
C)237°C
D)1581°C
E)-36.6°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
54
How many moles of gas are present in a 10.0 liter sample at STP?
A)224 moles
B)22.4 moles
C)10.0 moles
D)2.24 moles
E)0.446 moles
A)224 moles
B)22.4 moles
C)10.0 moles
D)2.24 moles
E)0.446 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
55
A sample of gas has a volume of 135 mL at 0.600 atm.What would be the volume if the pressure is decreased to 0.200 atm while temperature is held constant?
A)45.0 mL
B)101 mL
C)135 mL
D)180 mL
E)405 mL
A)45.0 mL
B)101 mL
C)135 mL
D)180 mL
E)405 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
56
Sketch a graph illustrating Charles's Law.Use a gas sample with a volume of 2.0 L at 0.75 atm and 27°C,and show at least two other points.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
57
Consider a sample of helium and a sample of neon,both at 30.0°C and 1.5 atm.Both samples have a volume of 5.0 liters.Which statement concerning these samples is not true?
A)Each sample contains the same number of atoms of gas.
B)Each sample weighs the same amount.
C)Each sample contains the same number of moles of gas.
D)The density of the neon is greater than the density of the helium.
E)none of the above
A)Each sample contains the same number of atoms of gas.
B)Each sample weighs the same amount.
C)Each sample contains the same number of moles of gas.
D)The density of the neon is greater than the density of the helium.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
58
How many grams of N2 are contained in an 11.2 liter sample at STP?
A)7.00
B)11.2
C)14.0
D)28.0
E)6.02 × 1023
A)7.00
B)11.2
C)14.0
D)28.0
E)6.02 × 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
59
How many grams of O2 are contained in a 25.0 L sample at 5.20 atm and 28.0°C?
A)1810 g
B)168 g
C)84.2 g
D)5.26 g
E)0.164 g
A)1810 g
B)168 g
C)84.2 g
D)5.26 g
E)0.164 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
60
A 65 mL sample of argon gas has a temperature of 325°C.What will the temperature be in °C when the volume of the gas is decreased to 25 mL at constant pressure?
A)65°C
B)125°C
C)-43°C
D)1280°C
E)641°C
A)65°C
B)125°C
C)-43°C
D)1280°C
E)641°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
61
The amount of energy involved in melting a substance without changing its temperature is called the
A)heat of vaporization.
B)calorie.
C)joule.
D)heat of fusion.
E)heat of combustion.
A)heat of vaporization.
B)calorie.
C)joule.
D)heat of fusion.
E)heat of combustion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
62
The total pressure of a mixture of gases is
A)obtained by multiplying the individual pressures by the number of moles and averaging.
B)the sum of the partial pressures of the components.
C)dependent only upon the pressure of the gas which is present to the greatest extent.
D)the product of the individual pressures.
E)none of these
A)obtained by multiplying the individual pressures by the number of moles and averaging.
B)the sum of the partial pressures of the components.
C)dependent only upon the pressure of the gas which is present to the greatest extent.
D)the product of the individual pressures.
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the pressure in a 1.00 liter container of methane,CH4,that contains 40.0 g of the gas at 25.0°C?
A)5.13 atm
B)61.0 atm
C)82.1 atm
D)979 atm
E)3920 atm
A)5.13 atm
B)61.0 atm
C)82.1 atm
D)979 atm
E)3920 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
64
At a high altitude water boils at 95°C instead of 100°C as at sea level because the
A)atmospheric pressure is greater.
B)atmospheric pressure is less.
C)climate is cooler.
D)vapor pressure of water is greater.
E)vapor pressure of water is less.
A)atmospheric pressure is greater.
B)atmospheric pressure is less.
C)climate is cooler.
D)vapor pressure of water is greater.
E)vapor pressure of water is less.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
65
In a gas mixture of 35% He and 65% O2 the total pressure is 800 mm Hg.What is the partial pressure of O2?
A)35 mm Hg
B)65 mm Hg
C)100 mm Hg
D)280 mm Hg
E)520 mm Hg
A)35 mm Hg
B)65 mm Hg
C)100 mm Hg
D)280 mm Hg
E)520 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
66
What volume will be occupied by 8.00 g of oxygen,O2,at STP?
A)5.60 L
B)11.2 L
C)22.4 L
D)13.4 L
E)4.00 L
A)5.60 L
B)11.2 L
C)22.4 L
D)13.4 L
E)4.00 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
67
If a 0.614 g sample of a gas maintains a pressure of 238 mm Hg when contained in a 1.0 L flask at 0.0°C,what is molar mass of the gas?
A)44 g/mol
B)120 g/mol
C)8.8 g/mol
D)22 g/mol
E)5 g/mol
A)44 g/mol
B)120 g/mol
C)8.8 g/mol
D)22 g/mol
E)5 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the total pressure of a mixture of He and H2 if the partial pressures are 320 mm Hg and 800 mm Hg respectively?
A)40 mm Hg
B)60 mm Hg
C)320 mm Hg
D)480 mm Hg
E)1120 mm Hg
A)40 mm Hg
B)60 mm Hg
C)320 mm Hg
D)480 mm Hg
E)1120 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
69
Calculate the volume of a 3.50 mol sample of carbon dioxide at 30.0°C and 0.900 atm.
A)8.54 × 10-4 L
B)2.20 L
C)9.58 L
D)96.7 L
E)421 L
A)8.54 × 10-4 L
B)2.20 L
C)9.58 L
D)96.7 L
E)421 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
70
The amount of energy associated with changing a liquid into a gas is called the
A)heat of vaporization.
B)heat of fusion.
C)heat of combustion.
D)joule.
E)calorie.
A)heat of vaporization.
B)heat of fusion.
C)heat of combustion.
D)joule.
E)calorie.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
71
List the five types of solids and at least two physical properties of each.Explain how the interparticle forces in that solid determine the properties you listed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the total pressure of a gaseous mixture that contains three gases with partial pressures of 0.845 atm,120 torr,and 210 mm Hg?
A)1175 torr
B)972 torr
C)0.411 atm
D)331 torr
A)1175 torr
B)972 torr
C)0.411 atm
D)331 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
73
As the temperature of a liquid is lowered,what happens to its vapor pressure?
A)Its vapor pressure drops.
B)Its vapor pressure rises.
C)The vapor pressure may rise or drop depending on the liquid.
D)Its vapor pressure is independent of temperature.
E)Its vapor pressure first rises then falls.
A)Its vapor pressure drops.
B)Its vapor pressure rises.
C)The vapor pressure may rise or drop depending on the liquid.
D)Its vapor pressure is independent of temperature.
E)Its vapor pressure first rises then falls.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
74
A mixture of the gases N2,O2,and He has a total pressure of 760 mm Hg.If the partial pressure of N2 is 90 mm Hg and of O2 is 270 mm Hg,what is the partial pressure of He?
A)1120 mm Hg
B)760 mm Hg
C)400 mm Hg
D)360 mm Hg
E)120 mm Hg
A)1120 mm Hg
B)760 mm Hg
C)400 mm Hg
D)360 mm Hg
E)120 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
75
A solid compound which has no definite crystalline structure and a poorly defined melting point is referred to as a(an)________ solid.
A)ionic
B)molecular
C)network covalent
D)amorphous
E)metallic
A)ionic
B)molecular
C)network covalent
D)amorphous
E)metallic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
76
The vapor pressure of a liquid
A)decreases with increasing temperature.
B)is independent of temperature.
C)is equal to one atmosphere at the normal boiling point.
D)cannot be measured.
A)decreases with increasing temperature.
B)is independent of temperature.
C)is equal to one atmosphere at the normal boiling point.
D)cannot be measured.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
77
Calculate the volume of 5.00 mol of helium at 120.°C and 1520 mm Hg.
A)323 liters
B)80.6 liters
C)0.106 liters
D)0.0324 liters
E)none of the above
A)323 liters
B)80.6 liters
C)0.106 liters
D)0.0324 liters
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
78
When a liquid sample is taken from sea level to a higher elevation,what happens to the external (atmospheric)pressure on the liquid and the boiling point of the liquid?
A)Both decrease.
B)Both increase.
C)Pressure goes down but boiling point goes up.
D)Pressure goes up but boiling point goes down.
A)Both decrease.
B)Both increase.
C)Pressure goes down but boiling point goes up.
D)Pressure goes up but boiling point goes down.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
79
List and describe two unique properties of water.Discuss the intermolecular force that causes these unusual properties.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
80
A sample of CO2 gas at 100.°C has a volume of 250.mL at 760.mm Hg.How many moles of CO2 are present?
A)0.00816 mol
B)0.0304 mol
C)6.20 mol
D)8.16 mol
E)23.14 mol
A)0.00816 mol
B)0.0304 mol
C)6.20 mol
D)8.16 mol
E)23.14 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck








