Deck 4: Thermodynamics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/192
العب
ملء الشاشة (f)
Deck 4: Thermodynamics
1
A CD player and its battery together do 500 kJ of work .The battery also releases 250 kJ of energy as heat and the CD player releases 50 kJ as heat due to friction from spinning.What is the change in internal energy of the system,
With the system regarded as the CD player alone? Assume that the battery does 500 kJ of work on the CD player,
Which then does the same amount of work on the surroundings.
A) -550 kJ
B) -50 kJ
C) -950 kJ
D) -800 kJ
E) +450 kJ
With the system regarded as the CD player alone? Assume that the battery does 500 kJ of work on the CD player,
Which then does the same amount of work on the surroundings.
A) -550 kJ
B) -50 kJ
C) -950 kJ
D) -800 kJ
E) +450 kJ
-50 kJ
2
An isolated system can exchange energy and small particles with the surroundings.
False
3
What is the total motional contribution to the molar internal energy of gaseous HCN?
A) 3RT
B) 3.5RT
C) 2.5RT
D) RT
E) 1.5RT
A) 3RT
B) 3.5RT
C) 2.5RT
D) RT
E) 1.5RT
C
4
When a gas expands into a vacuum,w = 0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
5
A CD player and its battery together do 500 kJ of work .The battery also releases 250 kJ of energy as heat,And the CD player releases 50 kJ as heat due to friction from spinning.What is the change in internal energy of the system,With the system regarded as the battery alone? Assume that the battery does 500 kJ of work on the CD player, Which then does the same amount of work on the surroundings.
A) +200 kJ
B) -800 kJ
C) -750 kJ
D) -50 kJ
E) -700 kJ
A) +200 kJ
B) -800 kJ
C) -750 kJ
D) -50 kJ
E) -700 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
6
If 2.00 mol of an ideal gas at 300 K and 3.00 atm expands from 6.00 L to 18.00 L and a final pressure of 1.20 atm in two steps: (1) the gas is cooled at constant volume until its pressure has fallen to 1.20 atm,And (2)It is heated and allowed to expand against a constant pressure of 1.20 atm until its volume reaches 18.00 L,Which of the following is correct?
A) w = 0 for step (1) and w =-1.46 kJ for step (2)
B) w = -4.57 kJ for the overall process
C) w = -6.03 kJ for the overall process
D) w = -4.57 kJ for step (1) and w = -1.46 kJ for step (2)
E) w = 0 for the overall process
A) w = 0 for step (1) and w =-1.46 kJ for step (2)
B) w = -4.57 kJ for the overall process
C) w = -6.03 kJ for the overall process
D) w = -4.57 kJ for step (1) and w = -1.46 kJ for step (2)
E) w = 0 for the overall process

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
7
A system had 150 kJ of work done on it and its internal energy increased by 60 kJ .
How much energy did the system gain or lose as heat?
A) The system lost 90 kJ of energy as heat.
B) The system lost 210 kJ of energy as heat.
C) The system gained 60 kJ of energy as heat.
D) The system gained 90 kJ of energy as heat.
E) The system gained 210 kJ of energy as heat.
How much energy did the system gain or lose as heat?
A) The system lost 90 kJ of energy as heat.
B) The system lost 210 kJ of energy as heat.
C) The system gained 60 kJ of energy as heat.
D) The system gained 90 kJ of energy as heat.
E) The system gained 210 kJ of energy as heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
8
How much work is done by a person of mass 185 kg who climbs a ladder to the top of his house, a total of 15.0 m?
A) 2.78 kJ
B) 12.3 kJ
C) 27.2 kJ
D) No work is done.
E) 121 kJ
A) 2.78 kJ
B) 12.3 kJ
C) 27.2 kJ
D) No work is done.
E) 121 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
9
A CD player and its battery together do 500 kJ of work .The battery also releases 250 kJ of energy as heat and the CD player releases 50 kJ as heat due to friction from spinning.What is the change in internal energy of the system,
With the system regarded as the battery and CD player together?
A) +200 kJ
B) -700 kJ
C) -800 kJ
D) -200 kJ
E) -750 kJ
With the system regarded as the battery and CD player together?
A) +200 kJ
B) -700 kJ
C) -800 kJ
D) -200 kJ
E) -750 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
10
Hot coffee in a vacuum flask (thermos) is an example of a(n)__________________ (open,closed,isolated)system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
11
Work is reported in joules; and 1 joule = ____________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
12
A closed system can exchange energy with the surroundings.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
13
A piece of a newly synthesized material of mass 25.0 g at 80.0 C is placed in a calorimeter containing 100.0 g of water at 20.0 C .If the final temperature of the system is 24.0 C,What is the specific heat capacity of this material?
A) 0.30 J.g-1.( C)-1
B) 7.46 J.g-1.( C)-1
C) 1.19 J.g-1.( C)-1
D) 4.76 J.g-1.( C)-1
E) 0.84 J.g-1.( C)-1
A) 0.30 J.g-1.( C)-1
B) 7.46 J.g-1.( C)-1
C) 1.19 J.g-1.( C)-1
D) 4.76 J.g-1.( C)-1
E) 0.84 J.g-1.( C)-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
14
A battery does 35 kJ of work driving an electric motor and 7 kJ of heat are released .What is the change in internal energy of the system?
A) -35 kJ
B) +42 kJ
C) -42 kJ
D) -28 kJ
E) +28 kJ
A) -35 kJ
B) +42 kJ
C) -42 kJ
D) -28 kJ
E) +28 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
15
A piece of a newly synthesized material of mass 12.0 g at 88.0 C is placed in a calorimeter containing 100.0 g of water at 20.0 C .If the final temperature of the system is 24.0 C,What is the specific heat capacity of this material?
A) 10.2 J.g-1.( C)-1
B) 1.58 J.g-1.( C)-1
C) 2.18 J.g-1.( C)-1
D) 9.50 J.g-1.( C)-1
E) 0.54 J.g-1.( C)-1
A) 10.2 J.g-1.( C)-1
B) 1.58 J.g-1.( C)-1
C) 2.18 J.g-1.( C)-1
D) 9.50 J.g-1.( C)-1
E) 0.54 J.g-1.( C)-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the total motional contribution to the molar internal energy of gaseous BF3?
A) RT
B) 3.5RT
C) 3RT
D) 1.5RT
E) 2.5RT
A) RT
B) 3.5RT
C) 3RT
D) 1.5RT
E) 2.5RT

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
17
If 2.00 mol of an ideal gas at 300 K and 3.00 atm expands from 6.00 L to 18.00 L and has a final pressure of 1.20 atm, isothermally and reversibly, Which of the following is correct?
A) w = -5.48 kJ, q = +5.48 kJ, U = 0
B) w = -3.65 kJ, q = +3.65 kJ, U = 0
C) w = +3.65 kJ, q = +3.65 kJ, U = +7.30 kJ
D) w = -5.48 kJ, q = -5.48 kJ, U = -11.0 kJ
E) w = +5.48 kJ, q = +5.48 kJ, U = +11.0 kJ
A) w = -5.48 kJ, q = +5.48 kJ, U = 0
B) w = -3.65 kJ, q = +3.65 kJ, U = 0
C) w = +3.65 kJ, q = +3.65 kJ, U = +7.30 kJ
D) w = -5.48 kJ, q = -5.48 kJ, U = -11.0 kJ
E) w = +5.48 kJ, q = +5.48 kJ, U = +11.0 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the total motional contribution to the molar internal energy of gaseous H2O at 25 C?
A) 6.19 kJ.mol-1
B) 7.43 kJ.mol-1
C) 3.72 kJ.mol-1
D) 12.4 kJ.mol-1
E) 2.48 kJ.mol-1
A) 6.19 kJ.mol-1
B) 7.43 kJ.mol-1
C) 3.72 kJ.mol-1
D) 12.4 kJ.mol-1
E) 2.48 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
19
If an isolated system contained +5 kJ of energy, after 100 years U =
A) The answer is impossible to determine
B) Slightly less than +5 kJ.
C) +5 kJ.
D) 0 kJ.
E) -5 kJ.
A) The answer is impossible to determine
B) Slightly less than +5 kJ.
C) +5 kJ.
D) 0 kJ.
E) -5 kJ.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
20
In a system composed of nitrogen gas in a cylinder fitted with a piston, when 2.00 kJ of energy is transferred as heat at an external pressure of 2.00 atm,
The nitrogen gas expands from 2.00 to 5.00 L against this constant pressure.What is U for the process?
A) -0.608 kJ
B) +1.39 kJ
C) +2.61 kJ
D) 0
E) -2.61 kJ
The nitrogen gas expands from 2.00 to 5.00 L against this constant pressure.What is U for the process?
A) -0.608 kJ
B) +1.39 kJ
C) +2.61 kJ
D) 0
E) -2.61 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
21
Calculate the change in internal energy when 2.50 kJ of energy is transferred as heat to 1.50 mol BF3 at 298 K and 1 atm at constant volume.
A) +1.00 kJ
B) +2.50 kJ
C) -1.50 kJ
D) -2.50 kJ
E) +1.50 kJ
A) +1.00 kJ
B) +2.50 kJ
C) -1.50 kJ
D) -2.50 kJ
E) +1.50 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
22
The combustion of 1 mole of octane, C8H18(l),To produce carbon dioxide and liquid water has Hr = -5471 kJ.mol-1 at 298 K.What is the change in internal energy for this reaction?
A) -5460 kJ.mol-1
B) -5482 kJ.mol-1
C) -5449 kJ.mol-1
D) -5471 kJ.mol-1
E) -5493 kJ.mol-1
A) -5460 kJ.mol-1
B) -5482 kJ.mol-1
C) -5449 kJ.mol-1
D) -5471 kJ.mol-1
E) -5493 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
23
Calculate the final temperature when 2.50 kJ of energy is transferred as heat to 1.50 mol BF3 at 298 K and 1 atm at constant volume.
A) 100 C
B) 80 C
C) 67 C
D) 125 C
E) 92 C
A) 100 C
B) 80 C
C) 67 C
D) 125 C
E) 92 C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
24
The combustion of 1 mole of ethanol, C2H5OH(l),In a bomb calorimeter released 1240 kJ of heat.The products of the reaction are carbon dioxide and liquid water.What is the enthalpy change for this reaction?
A) -1245 kJ.mol-1
B)-1235 kJ.mol-1
C) +1235 kJ.mol-1
D) +1240 kJ.mol-1
E) -1240 kJ.mol-1
A) -1245 kJ.mol-1
B)-1235 kJ.mol-1
C) +1235 kJ.mol-1
D) +1240 kJ.mol-1
E) -1240 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
25
A reaction known to release 2.00 kJ of heat takes place in a calorimeter containing 0.200 L of solution and the temperature rose by 4.46 C .When 100 mL of nitric acid and 100 mL of sodium hydroxide were mixed in the same calorimeter,The temperature rose 2.01 C.What is the heat output for the neutralization reaction?
A) 0.0186 k.J.( C)-1
B) 0.448 k.J.( C)-1
C) 0.816 k.J.( C)-1
D) 17.9 k.J.( C)-1
E) 0.901 k.J.( C)-1
A) 0.0186 k.J.( C)-1
B) 0.448 k.J.( C)-1
C) 0.816 k.J.( C)-1
D) 17.9 k.J.( C)-1
E) 0.901 k.J.( C)-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
26
Calculate the change in internal energy when 2.50 kJ of energy is transferred as heat to 1.50 mol BF3 at 298 K and 1 atm at constant pressure.
A) +1.56 kJ
B) +2.50 kJ
C) 1.87 kJ
D) -2.50 kJ
E) -1.87 kJ
A) +1.56 kJ
B) +2.50 kJ
C) 1.87 kJ
D) -2.50 kJ
E) -1.87 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
27
Calculate the change in internal energy when 2.50 kJ of energy is transferred as heat to 1.50 mol N2 at 298 K and 1 atm at constant pressure.
A) +2.14 kJ
B) -1.79 kJ
C) +2.50 kJ
D)-2.50 kJ
E) +1.79 kJ
A) +2.14 kJ
B) -1.79 kJ
C) +2.50 kJ
D)-2.50 kJ
E) +1.79 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
28
How much heat is required to vaporize 50.0 g of water if the initial temperature of the water is 25.0 C and the water is heated to its boiling point, where it is converted to steam? The specific heat capacity of water is 4.18 J.( C)-1g-1 and the standard enthalpy of vaporization of water at its boiling point is 40.7 kJ.mol-1.
A) 169 kJ
B) 64.2 kJ
C) 40.7 kJ
D) 193 kJ
E) 23.5 kJ
A) 169 kJ
B) 64.2 kJ
C) 40.7 kJ
D) 193 kJ
E) 23.5 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
29
A reaction known to release 4.00 kJ of heat takes place in a calorimeter containing 0.200 L of solution and the temperature rose by 6.14 C .When 200 mL of hydrochloric acid was added to a small piece of calcium carbonate in the same calorimeter,The temperature rose by 4.25 C.What is the heat output for this reaction?
A) 0.0257k.J.( C)-1
B) 0.941 k.J.( C)-1
C) 0.651 k.J.( C)-1
D) 2.77 k.J.( C)-1
E) 2.12 k.J.( C)-1
A) 0.0257k.J.( C)-1
B) 0.941 k.J.( C)-1
C) 0.651 k.J.( C)-1
D) 2.77 k.J.( C)-1
E) 2.12 k.J.( C)-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
30
Calculate the final temperature when 2.50 kJ of energy is transferred as heat to 1.50 mol N2 at 298 K and 1 atm at constant volume.
A) 80 C
B) 145 C
C) 159 C
D) 120 C
E) 105 C
A) 80 C
B) 145 C
C) 159 C
D) 120 C
E) 105 C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
31
The combustion of 1 mole of ethanol, C2H5OH(l),To produce carbon dioxide and gaseous water has Hr = -1235 kJ.mol-1 at 298 K.What is the change in internal energy for this reaction?
A) -1237 kJ.mol-1
B) -1240 kJ.mol-1
C) -1230 kJ.mol-1
D) -1247 kJ.mol-1
E) -1223 kJ.mol-1
A) -1237 kJ.mol-1
B) -1240 kJ.mol-1
C) -1230 kJ.mol-1
D) -1247 kJ.mol-1
E) -1223 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
32
Calculate the enthalpy change that occurs when 1.00 kg of acetone condenses at its boiling point (329.4 K) .The standard enthalpy of vaporization of acetone is29.1 kJ.mol-1.
A) -502 kJ
B)-29.1 kJ
C) -2.91 * 104 kJ
D) +502 kJ
E) +29.1 kJ
A) -502 kJ
B)-29.1 kJ
C) -2.91 * 104 kJ
D) +502 kJ
E) +29.1 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
33
When a solution of 1.691 g of silver nitrate is mixed with an excess of sodium chloride in a calorimeter of heat capacity 216 J.( C)-1, the temperature rises 3.03 C.What is the reaction enthalpy?
A) -65.7 kJ.mol-1
B) -0.654 kJ.mol-1
C) +111 kJ.mol-1
D) +65.7 kJ.mol-1
E) +0.654 kJ.mol-1
A) -65.7 kJ.mol-1
B) -0.654 kJ.mol-1
C) +111 kJ.mol-1
D) +65.7 kJ.mol-1
E) +0.654 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following is not a state function?
A) H
B) T
C) U
D) S
E) None; they are all state functions.
A) H
B) T
C) U
D) S
E) None; they are all state functions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
35
Calculate the enthalpy change that occurs when 1 lb (454 g) of mercury freezes at its freezing point (234.3 K).The standard enthalpy of fusion of mercury is 2.29kJ.mol-1.
A) -2.29 kJ
B) -1.04 * 103 kJ
C) +5.18 kJ
D) +2.29 kJ
E) -5.18 kJ
A) -2.29 kJ
B) -1.04 * 103 kJ
C) +5.18 kJ
D) +2.29 kJ
E) -5.18 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
36
Calculate the final temperature when 2.50 kJ of energy is transferred as heat to 1.50 mol BF3 at 298 K and 1 atm at constant pressure.
A) 82 C
B) 80 C
C) 159 C
D) 75 C
E) 92 C
A) 82 C
B) 80 C
C) 159 C
D) 75 C
E) 92 C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
37
Calculate the standard enthalpy of vaporization of liquid bromine if the standard enthalpy of sublimation of solid bromine is +40.1 kJ.mol-1 and the standard enthalpy of fusion of solid bromine is +10.6 kJ.mol-1
A) -50.7 kJ.mol-1
B) -29.5 kJ.mol-1
C) +50.7 kJ.mol-1
D) +14.8 kJ.mol-1
E) +29.5 kJ.mol-1
A) -50.7 kJ.mol-1
B) -29.5 kJ.mol-1
C) +50.7 kJ.mol-1
D) +14.8 kJ.mol-1
E) +29.5 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
38
Calculate the final temperature when 2.50 kJ of energy is transferred as heat to 1.50 mol N2 at 298 K and 1 atm at constant pressure.
A) 75 C
B) 159 C
C) 80 C
D) 82 C
E) 92 C
A) 75 C
B) 159 C
C) 80 C
D) 82 C
E) 92 C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
39
Calculate the change in internal energy when 2.50 kJ of energy is transferred as heat to 1.50 mol N2 at 298 K and 1 atm at constant volume.
A) -2.50 kJ
B) +2.50 kJ
C) +1.00 kJ
D) -1.50 kJ
E) +1.50 kJ
A) -2.50 kJ
B) +2.50 kJ
C) +1.00 kJ
D) -1.50 kJ
E) +1.50 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
40
The combustion of one mole of octane, C8H18(l), In a bomb calorimeter released 5460 kJ of heat at 298 K.The products of the reaction are carbon dioxide and liquid water.What is the enthalpy change for this reaction?
A) +5471 kJ.mol-1
B) -5460 kJ.mol-1
C) -5471 kJ.mol-1
D) -5449 kJ.mol-1
E) +5460 kJ.mol-1
A) +5471 kJ.mol-1
B) -5460 kJ.mol-1
C) -5471 kJ.mol-1
D) -5449 kJ.mol-1
E) +5460 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
41
Calculate the lattice enthalpy of calcium oxide from the following data.
Enthalpy of formation of Ca(g): +178 kJ.mol-1
First ionization energy of Ca(g): +590 kJ.mol-1
Second ionization energy of Ca(g): +1150 kJ.mol-1
Enthalpy of formation of O(g): +249 kJ.mol-1
First electron affinity of O(g): +141 ( H = kJ.mol-1
Second electron affinity of O(g): -844 H = +844)kJ.mol-1
Enthalpy of formation of CaO(s): -635 kJ.mol-1
A) 1817 kJ.mol-1
B) 1391 kJ.mol-1
C) 2235 kJ.mol-1
D) 3754 kJ.mol-1
E) 3505 kJ.mol-1
Enthalpy of formation of Ca(g): +178 kJ.mol-1
First ionization energy of Ca(g): +590 kJ.mol-1
Second ionization energy of Ca(g): +1150 kJ.mol-1
Enthalpy of formation of O(g): +249 kJ.mol-1
First electron affinity of O(g): +141 ( H = kJ.mol-1
Second electron affinity of O(g): -844 H = +844)kJ.mol-1
Enthalpy of formation of CaO(s): -635 kJ.mol-1
A) 1817 kJ.mol-1
B) 1391 kJ.mol-1
C) 2235 kJ.mol-1
D) 3754 kJ.mol-1
E) 3505 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
42
The standard enthalpy of formation of NaCl(s) is -411 kJ/mol.In a Born-Haber cycle for the formation of NaCl(s),Which enthalpy change(s)
Is/are endothermic?
A) the lattice enthalpy of NaCl(s)
B) the electron affinity of chlorine
C) the reverse of the lattice enthalpy of NaCl(s)
D) All of the enthalpy changes are endothermic except for the standard enthalpy of formation of NaCl(s).
Is/are endothermic?
A) the lattice enthalpy of NaCl(s)
B) the electron affinity of chlorine
C) the reverse of the lattice enthalpy of NaCl(s)
D) All of the enthalpy changes are endothermic except for the standard enthalpy of formation of NaCl(s).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
43
Calculate the standard reaction enthalpy for the reaction.
A) -935 kJ.mol-1
B) -1119 kJ.mol-1
C)-151 kJ.mol-1
D) -59 kJ.mol-1
E) -243 kJ.mol-1
A) -935 kJ.mol-1
B) -1119 kJ.mol-1
C)-151 kJ.mol-1
D) -59 kJ.mol-1
E) -243 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
44
The lattice enthalpy of calcium oxide is the energy change for the reaction
A) CaO(s) Ca(g) + O(g)
B) CaO(s) Ca(g) + ½O2(g)
C) Ca(g) + O(g) CaO(g)
D) CaO(s) Ca2+(g) + O2-(g)
E) Ca(s) + ½O2(g) CaO(s)
A) CaO(s) Ca(g) + O(g)
B) CaO(s) Ca(g) + ½O2(g)
C) Ca(g) + O(g) CaO(g)
D) CaO(s) Ca2+(g) + O2-(g)
E) Ca(s) + ½O2(g) CaO(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
45
Calculate the lattice enthalpy of potassium chloride given the following enthalpy data.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
46
Calculate the standard enthalpy of combustion of ethanol at 298 K from standard enthalpy of formation data.
A) -957.0 kJ.mol-1
B) -1367 kJ.mol-1
C) -687.5 kJ.mol-1
D) -1922 kJ.mol-1
E) -401.7 kJ.mol-1
A) -957.0 kJ.mol-1
B) -1367 kJ.mol-1
C) -687.5 kJ.mol-1
D) -1922 kJ.mol-1
E) -401.7 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
47
Given the standard reaction enthalpies below:
N2(g)+ O2 2NO(g)
H° = +180.5 kJ.mol-1
2NO2(g) N2(g)+ 2O2(g)
H° = -66.4 kJ.mol-1
Calculate the standard reaction enthalpy for the oxidation of nitric oxide to nitrogen dioxide,
Shown below:
2NO(g)+ O2(g) 2NO2(g)
A) .-114.1 kJ.mol-1
B) -246.9 kJ.mol-1 .
C)-114.1 kJ.mol-1
D). -294.6 kJ.mol-1 .
E) - 246.9 kJ.mol-1 .
N2(g)+ O2 2NO(g)
H° = +180.5 kJ.mol-1
2NO2(g) N2(g)+ 2O2(g)
H° = -66.4 kJ.mol-1
Calculate the standard reaction enthalpy for the oxidation of nitric oxide to nitrogen dioxide,
Shown below:
2NO(g)+ O2(g) 2NO2(g)
A) .-114.1 kJ.mol-1
B) -246.9 kJ.mol-1 .
C)-114.1 kJ.mol-1
D). -294.6 kJ.mol-1 .
E) - 246.9 kJ.mol-1 .

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
48
Calculate the standard reaction enthalpy for the following reaction.
A) +155.5 kJ.mol-1
B) + 216 kJ.mol-1
C) +412.1 kJ.mol-1
D) +42.0 kJ.mol-1
E) +206.1 kJ.mol-1
A) +155.5 kJ.mol-1
B) + 216 kJ.mol-1
C) +412.1 kJ.mol-1
D) +42.0 kJ.mol-1
E) +206.1 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
49
If the standard enthalpy of combustion of ethanol, C2H5OH(l),At 298 K is -1368 kJ.mol-1 ,What is the standard enthalpy of formation of ethanol? The standard enthalpies of formation of carbon dioxide and liquid water are -393.51 and -285.83 kJ.mol-1 ,Respectively.
A) +688.7 kJ.mol-1
B) -344.3 kJ.mol-1
C) +276.5 kJ.mol-1
D) -276.5 kJ.mol-1
E) -688.7 kJ.mol-1
A) +688.7 kJ.mol-1
B) -344.3 kJ.mol-1
C) +276.5 kJ.mol-1
D) -276.5 kJ.mol-1
E) -688.7 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
50
If the standard enthalpy of combustion of octane,
C8H18(l),at 298 K is -5471 kJ.mol-1 calculate the standard enthalpy of formation of octane.The standard enthalpies of formation of carbon dioxide and liquid water are -393.51 and -285.83 kJ.mol-1 ,respectively.
C8H18(l),at 298 K is -5471 kJ.mol-1 calculate the standard enthalpy of formation of octane.The standard enthalpies of formation of carbon dioxide and liquid water are -393.51 and -285.83 kJ.mol-1 ,respectively.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
51
If the standard reaction enthalpy for the reaction
NO2(g) NO(g)+ O(g)
Is needed,Calculate it from the standard enthalpy of formation of ozone,+142.7kJ.mol-1 ,And from the reactions listed below:
O2(g) 2O(g)
H° = +498.4 kJ.mol-1
NO(g)+ O3(g) NO2(g)+ O2(g)
H° = -200 kJ.mol-1
A) +355 kJ.mol-1
B) +555 kJ.mol-1
C) +192 kJ.mol-1
D) +592 kJ.mol-1
E) +306 kJ.mol-1
NO2(g) NO(g)+ O(g)
Is needed,Calculate it from the standard enthalpy of formation of ozone,+142.7kJ.mol-1 ,And from the reactions listed below:
O2(g) 2O(g)
H° = +498.4 kJ.mol-1
NO(g)+ O3(g) NO2(g)+ O2(g)
H° = -200 kJ.mol-1
A) +355 kJ.mol-1
B) +555 kJ.mol-1
C) +192 kJ.mol-1
D) +592 kJ.mol-1
E) +306 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
52
Calculate the lattice enthalpy of silver chloride from the following data.
Enthalpy of formation of Ag(g): +284 kJ.mol-1
First ionization energy of Ag(g): +731 kJ.mol-1
Enthalpy of formation of Cl(g): +122 kJ.mol-1
Electron affinity of Cl(g): +349 ( H = -349)
kJ.mol-1
Enthalpy of formation of AgCl(s): -127 kJ.mol-1
A) 1613 kJ.mol-1
B) 915 kJ.mol-1
C) 1037 kJ.mol-1
D) 1359 kJ.mol-1
E) 661 kJ.mol-1
Enthalpy of formation of Ag(g): +284 kJ.mol-1
First ionization energy of Ag(g): +731 kJ.mol-1
Enthalpy of formation of Cl(g): +122 kJ.mol-1
Electron affinity of Cl(g): +349 ( H = -349)
kJ.mol-1
Enthalpy of formation of AgCl(s): -127 kJ.mol-1
A) 1613 kJ.mol-1
B) 915 kJ.mol-1
C) 1037 kJ.mol-1
D) 1359 kJ.mol-1
E) 661 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
53
Calculate the lattice enthalpy of potassium fluoride from the following data.
Enthalpy of formation of K(g): +89 kJ.mol-1
First ionization energy of K(g): +418 kJ.mol-1
Enthalpy of formation of F(g): +79 kJ.mol-1
Electron affinity of F(g): +328 ( H = kJ.mol-1
Enthalpy of formation of KF(s): -567 kJ.mol-1
A) 1481 kJ.mol-1
B) 825 kJ.mol-1
C) 904 kJ.mol-1
D) 497 kJ.mol-1
E) 347 kJ.mol-1
Enthalpy of formation of K(g): +89 kJ.mol-1
First ionization energy of K(g): +418 kJ.mol-1
Enthalpy of formation of F(g): +79 kJ.mol-1
Electron affinity of F(g): +328 ( H = kJ.mol-1
Enthalpy of formation of KF(s): -567 kJ.mol-1
A) 1481 kJ.mol-1
B) 825 kJ.mol-1
C) 904 kJ.mol-1
D) 497 kJ.mol-1
E) 347 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
54
What equation corresponds to the standard enthalpy of formation of gaseous hydrogen atoms,which,at 298 K is +217 kJ.mol-1 ?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
55
What mass of propane, C3H8(g),Must be burned to supply 2580 kJ of heat? The standard enthalpy of combustion of propane at 298 K is -2220 kJ.mol-1
A) 25.6 g
B) 51.2 g
C) 102 g
D) 75.9 g
E) 37.9 g
A) 25.6 g
B) 51.2 g
C) 102 g
D) 75.9 g
E) 37.9 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
56
The standard enthalpy of formation of ammonium perchlorate at 298 K is -295.31 kJ.mol-1 .Write the equation that corresponds to this value.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
57
Match the values with the correct enthalpy change.
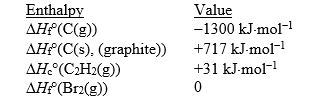
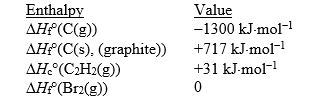

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
58
What mass of ethanol, C2H5OH(l),Must be burned to supply 500 kJ of heat? The standard enthalpy of combustion of ethanol at 298 K is -1368 kJ.mol-1
A) 126 g
B) 2.74 g
C) 16.8 g
D) 10.9 g
E) 29.7 g
A) 126 g
B) 2.74 g
C) 16.8 g
D) 10.9 g
E) 29.7 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
59
Calculate the standard enthalpy of combustion of butane, C4H10(g),
at 298 K from standard enthalpy of formation data.
at 298 K from standard enthalpy of formation data.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
60
The lattice enthalpy of calcium bromide is the energy change for the reaction
A) CaBr2(s) Ca(g) + 2Br(g)
B) CaBr2(s) Ca2+(g) + 2Br-(g)
C) Ca(s) + Br2(l) CaBr2(s)
D) CaBr2(s) Ca(g) + Br2(g)
E) Ca(g) + 2Br(g) CaBr2(g)
A) CaBr2(s) Ca(g) + 2Br(g)
B) CaBr2(s) Ca2+(g) + 2Br-(g)
C) Ca(s) + Br2(l) CaBr2(s)
D) CaBr2(s) Ca(g) + Br2(g)
E) Ca(g) + 2Br(g) CaBr2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
61
The standard enthalpy of formation of ammonia gas is -46.11 kJ.mol-1 at 298 K .What is the standard reaction enthalpy for the Haber process at 500 C? The molar heat capacities of nitrogen,Hydrogen,And ammonia are29.12,28.82,And 35.06J.k.mol-1 respectively.
A) -97.65 kJ.mol-1
B) -113.81 kJ.mol-1
C) -56.91 kJ.mol-1
D)-92.22 kJ.mol-1
E) -3.09 kJ.mol-1
A) -97.65 kJ.mol-1
B) -113.81 kJ.mol-1
C) -56.91 kJ.mol-1
D)-92.22 kJ.mol-1
E) -3.09 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
62
The molar heat capacity of a monatomic ideal gas is independent of temperature and pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
63
The formation of solid calcium oxide from a gas of its ions is an exothermic process and is equal to the reverse of the lattice enthalpy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
64
The heat flow for the reactionC2H6(g)+ 3.5O2(g) 2CO2(g)+ 3H2O(l)Measured in a bomb calorimeter is -1553.5 kJ/mol at 298 K.At this temperature, U is
A) -1553.5 kJ/mol
B) -1552.3 kJ/mol
C) -1547.3 kJ/mol
D) -1559.7 kJ/mol
A) -1553.5 kJ/mol
B) -1552.3 kJ/mol
C) -1547.3 kJ/mol
D) -1559.7 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
65
Use the following information to determine the standard enthalpy of formation of NH3(g).
N-H bond enthalpy = 390 kJ.mol-1
Hf (H(g))= 217.9 kJ.mol-1
Hf (N(g))= 472.6 kJ.mol-1
A) -44 kJ.mol-1
B) -691 kJ.mol-1
C) -516 kJ.mol-1
D) -83 kJ.mol-1
E) -1170 kJ.mol-1
N-H bond enthalpy = 390 kJ.mol-1
Hf (H(g))= 217.9 kJ.mol-1
Hf (N(g))= 472.6 kJ.mol-1
A) -44 kJ.mol-1
B) -691 kJ.mol-1
C) -516 kJ.mol-1
D) -83 kJ.mol-1
E) -1170 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
66
Give Hr values for each of the following reactions from the information below.
Hf (HCl(g))= -92.31 kJ.mol-1
H-Cl bond enthalpy = +431 kJ.mol-1
O-H bond enthalpy = +463 kJ.mol-1
Hf (H2O(g))= -241.8 kJ.mol-1
(a)2H(g)+ O(g) H2O(g)
(b)H2(g)+ Cl2(g) 2HCl(g)
(c)H(g)+ Cl(g) HCl(g)
Hf (HCl(g))= -92.31 kJ.mol-1
H-Cl bond enthalpy = +431 kJ.mol-1
O-H bond enthalpy = +463 kJ.mol-1
Hf (H2O(g))= -241.8 kJ.mol-1
(a)2H(g)+ O(g) H2O(g)
(b)H2(g)+ Cl2(g) 2HCl(g)
(c)H(g)+ Cl(g) HCl(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
67
Calculate the average H-S bond enthalpy in H2S(g) given the standard enthalpies of formation for H2S(g),H(g),And S(g):-20.1,218,And 223kJ.mol-1 ,Respectively.
A) 340 kJ.mol-1
B) 231 kJ.mol-1
C) 10.1 kJ.mol-1
D) 679 kJ.mol-1
E) 461 kJ.mol-1
A) 340 kJ.mol-1
B) 231 kJ.mol-1
C) 10.1 kJ.mol-1
D) 679 kJ.mol-1
E) 461 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
68
Calculate the standard enthalpy of formation of bicyco[1.1.0]butane (shown below) ![<strong>Calculate the standard enthalpy of formation of bicyco[1.1.0]butane (shown below) given the standard enthalpies of formation of C(g)And H(g),717 and 218 kJ.mol<sup>-</sup><sup>1 </sup>,Respectively,And the average C-H and C-C bond enthalpies,412 and 348 kJ.mol<sup>-1</sup>,Respectively.</strong> A) +175 kJ.mol<sup>-</sup><sup>1 </sup> B) -472 kJ.mol<sup>-</sup><sup>1 </sup> C) +312 kJ.mol<sup>-</sup><sup>1 </sup> D) -36 kJ.mol<sup>-</sup><sup>1 </sup> E) -124 kJ.mol<sup>-</sup><sup>1 </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1193/11ea7e78_6406_96ab_9a0a_ad814ed11d0c_TB1193_00.jpg) given the standard enthalpies of formation of C(g)And H(g),717 and 218 kJ.mol-1 ,Respectively,And the average C-H and C-C bond enthalpies,412 and 348 kJ.mol-1,Respectively.
given the standard enthalpies of formation of C(g)And H(g),717 and 218 kJ.mol-1 ,Respectively,And the average C-H and C-C bond enthalpies,412 and 348 kJ.mol-1,Respectively.
A) +175 kJ.mol-1
B) -472 kJ.mol-1
C) +312 kJ.mol-1
D) -36 kJ.mol-1
E) -124 kJ.mol-1
![<strong>Calculate the standard enthalpy of formation of bicyco[1.1.0]butane (shown below) given the standard enthalpies of formation of C(g)And H(g),717 and 218 kJ.mol<sup>-</sup><sup>1 </sup>,Respectively,And the average C-H and C-C bond enthalpies,412 and 348 kJ.mol<sup>-1</sup>,Respectively.</strong> A) +175 kJ.mol<sup>-</sup><sup>1 </sup> B) -472 kJ.mol<sup>-</sup><sup>1 </sup> C) +312 kJ.mol<sup>-</sup><sup>1 </sup> D) -36 kJ.mol<sup>-</sup><sup>1 </sup> E) -124 kJ.mol<sup>-</sup><sup>1 </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1193/11ea7e78_6406_96ab_9a0a_ad814ed11d0c_TB1193_00.jpg) given the standard enthalpies of formation of C(g)And H(g),717 and 218 kJ.mol-1 ,Respectively,And the average C-H and C-C bond enthalpies,412 and 348 kJ.mol-1,Respectively.
given the standard enthalpies of formation of C(g)And H(g),717 and 218 kJ.mol-1 ,Respectively,And the average C-H and C-C bond enthalpies,412 and 348 kJ.mol-1,Respectively.A) +175 kJ.mol-1
B) -472 kJ.mol-1
C) +312 kJ.mol-1
D) -36 kJ.mol-1
E) -124 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
69
An isothermal change is one that occurs at a constant temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
70
At constant pressure and temperature, which of the following statements is true for the reaction:
N2O5(s) 2NO2(g)+ ½O2(g),
Hr = 109.5 kJ.mol-1 at 298 K.
A) H is independent of the physical states of the reactants.
B) H = U
C) w = 0
D) H < U
E) H > U
N2O5(s) 2NO2(g)+ ½O2(g),
Hr = 109.5 kJ.mol-1 at 298 K.
A) H is independent of the physical states of the reactants.
B) H = U
C) w = 0
D) H < U
E) H > U

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
71
For the reaction CO2(aq) CO2(g),
Hr = 19.4 kJ.mol-1 at 298.At constant temperature and pressure,Which of the following statements is true?
A) w = 0
B) U = 2.48 kJ
C) H < U
D) H = U
E) H > U
Hr = 19.4 kJ.mol-1 at 298.At constant temperature and pressure,Which of the following statements is true?
A) w = 0
B) U = 2.48 kJ
C) H < U
D) H = U
E) H > U

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
72
Calculate the H-Br bond enthalpy given the standard enthalpies of formation for HBr(g), H(g),And Br(g): -36.2,218, And112kJ.mol-1 ,Respectively.
A) 320 kJ.mol-1
B) 366 kJ.mol-1
C) 124 kJ.mol-1
D) 284 kJ.mol-1
E) 196 kJ.mol-1
A) 320 kJ.mol-1
B) 366 kJ.mol-1
C) 124 kJ.mol-1
D) 284 kJ.mol-1
E) 196 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
73
Calculate the Br-Br bond enthalpy given the standard enthalpies of formation for Br2(g) and Br(g),30.7 and 112 kJ.mol-1 ,Respectively.
A) 193 kJ.mol-1
B) 255 kJ.mol-1
C) 143 kJ.mol-1
D) 81 kJ.mol-1
E) 30.7 kJ.mol-1
A) 193 kJ.mol-1
B) 255 kJ.mol-1
C) 143 kJ.mol-1
D) 81 kJ.mol-1
E) 30.7 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
74
The enthalpy of sublimation of a substance is related to its enthalpy of vaporization and enthalpy of fusion by the equation,
Hsublimation = Hvaporization - Hfusion.
Hsublimation = Hvaporization - Hfusion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
75
Determine Hf (HCl(g)) from the following data.
H-Cl bond enthalpy = +431 kJ.mol-1
Hf (H(g))= +217.9 kJ.mol-1
Hf (Cl(g))= +121.4 kJ.mol-1
A) +92 kJ.mol-1
B) -261 kJ.mol-1
C)-431 kJ.mol-1
D) +431 kJ.mol-1
E) -92 kJ.mol-1
H-Cl bond enthalpy = +431 kJ.mol-1
Hf (H(g))= +217.9 kJ.mol-1
Hf (Cl(g))= +121.4 kJ.mol-1
A) +92 kJ.mol-1
B) -261 kJ.mol-1
C)-431 kJ.mol-1
D) +431 kJ.mol-1
E) -92 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
76
Calculate the standard enthalpy of formation of potassium chloride given the following enthalpy data.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
77
The standard enthalpy of formation of gaseous water is -241.82 kJ.mol-1 at 298 K .Estimate this value at 370 K.The molar heat capacities of hydrogen,Oxygen,And gaseous waterare 28.82,29.36,And 33.58J.k.mol-1
A) -241.82 kJ.mol-1
B) -243.25 kJ.mol-1
C) -242.54 kJ.mol-1
D) -240.39 kJ.mol-1
E) -241.10 kJ.mol-1
A) -241.82 kJ.mol-1
B) -243.25 kJ.mol-1
C) -242.54 kJ.mol-1
D) -240.39 kJ.mol-1
E) -241.10 kJ.mol-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
78
Combustion reactions can be exothermic or endothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
79
For any isothermal process, U > 0 for an ideal gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck
80
The formation of solid calcium chloride from a gas of its ions is an exothermic process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 192 في هذه المجموعة.
فتح الحزمة
k this deck








